Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
Okay; I'll admit that nearly any selenium species tested—be it selenite, selenate, methylselenol, dimethylselenide, L-selenomethionine, D-selenomethionine, selenocysteine, methylselenocysteine, etc.—inhibits proliferation of cancer cells in vitro and tumors in vivo to some degree, but L-selenomethionine seems to be the most effective gram for gram. Nearly every selenium species seems have a different mechanism of action and many have been formally proposed. I had concentrated on the polyamine pathway for L-selenomethionine inhibition explicitly because it is intuitive, biochemically-plausible, and has been proven to occur.⁽¹⁾ It has been known since the '60s that polyamines bind dNA⁽²⁾, and shortly thereafter they've been shown to catalyze dNA replication via PCR—an aqueous system using only enzymes, nucleotides, and heat (polyamines being optional).⁽³⁾ Later investigations had revealed that spermine lowers the energy barrier from the B-dNA configuration to the Z-dNA configuration,⁽⁴⁾ literally transforming the normal right-handed helix into one left-handed. Polyamines also have an increased affinity for 'CpG islands,' areas relatively-rich in 5-methylctyosine that also tend to be housekeeping genes.⁽⁵⁾ Polyamines so reliably catalyze dNA replication that they can rightly be considered a small-molecule transcription factor.
Thomas Baine's doctoral dissertation goes a long way towards understanding the enigmatic effects of selenium on cancer cells.⁽⁶⁾ While aiming to elucidate the primary mechanism of organic selenium species, Doctor Baines had analyzed the effects of selenomethionine and methylselenocysteine on polyamines and prostaglandin E₂. As reliable cancer cell proliferents: these two molecules seem a logical focus and Dr. Baines had previously been familiar with selenomethionine's polyamine inhibition; after all, he had co-authored the Redman article (not that Redman article, the other Redman article).⁽⁷⁾ He had given rats low parts per million doses in their food―
――――――――――――――――――――――――――――――――――――――――――――――――――
It is important to give selenomethionine in food and not water, or you could end-up with results like this:⁽⁸⁾

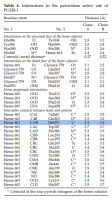
Doctor Niall Corcoran, a urologist from Australia, had given eight groups or rats eight different selenium species in their drinking water. Upon seeing the results, he trumpets 'inorganic selenium' (sodium selenide) as being the most effective selenium species. What Doctor Corcoran failed to consider, however, is the very low solubility of selenomethionine:⁽⁹⁾
Methionine has a density of 1.34g/cm³ yet selenomethionine weights 49.3% more. Although Corcoran's flask could initially hold 3·ppm of selenomethionine in solution—as reported—it could potentially form a density gradient over time, depending of course on the time passed between mixing and mouse-administration. Accurate solubilities of selenomethionine do not seem to be commonly reported, and even Sigma–Aldrich lazily uses methionine's solubility to represent that of selenomethionine—as seen here and here—despite it being over three times less.⁽⁹⁾ Although the bottom of Corcoran's volumetric flask most certainly had 3·ppm of selenomethionine, and probably much more, the concentration actually poured in the mouses tongue-activated ball-bearing water dispenser could have been much lower. Since all other studies I've read show selenomethionine to be much more active than selenide, I think it's fair to conclude that the results reported by Doctor Niall Corcoran—a urologist from Australia—had been confounded by water solubility considerations.
――――――――――――――――――――――――――――――――――――――――――――――――――
...and had found the expected cancer reductions. In isolated human cells, selenomethionine had caused reduced cyclooxygenase-2 protein levels after six days despite invariable mRNA levels (in HT-29 cells). Prostaglandin E₂ was correspondingly reduced, and these results were so significant that he'd decided to focus on it.
Yet he doesn't explain it, despite a large proportion of his 146-page dissertation being devoted to the 'discussion.' However, cyclooxygenase-2 does have a methionine in it's catalytic domain at position #131,⁽¹⁰⁾ and a six-day lag period is consistent with enzyme turnover & new protein synthesis.

 click to embiggen
click to embiggen
Although superoxide or oxygen are commonly assumed to be the other substrate besides arachidonate, detailed studies reveal that peroxynitrite is actually responsible.⁽¹²⁾ Peroxynitrite (ONOO⁻) is formed from the spontaneous interaction of superoxide (Ȯ₂⁻) with nitric oxide (ṄO), and is generally considered the most damaging reactive nitrogen species known. Inducible nitric oxide synthase (iNOS) reliably increases prostaglandin formation, and nitric oxide seems to be why. Peroxynitrite is perhaps known best for its tyrosine adduct, yet it has been shown to interact with lysine and methionine.⁽¹³⁾ Lysine-294 sits at the top of cyclooxygenase's heme ring, while (Se)methionine-131 sits on the bottom:⁽¹¹⁾

Cytochrome C peroxidase is a heme protein with a core iron atom axially-ligated on the underside by a thiol, a cysteine in this case.⁽¹⁴⁾ Since demethylated methionine is also a thiol, as homocysteine, I think it's reasonable to assume that cyclooxygenase's heme iron is complexed in the same way. The homocysteine of cyclooxygenase-2 could be situtated in a way analogous to the thiol–Fe²⁺ interaction of cytochrome C peroxidase (left), and could perhaps be better visualized by considering how cobalamin's central cobalt is complexed (right).


Perhaps heme enzymes normally axially-ligated through methionine exhibit a reduced activity when substituted by selenomethionine? This is not a radical proposal, and mutagenic studies have shown selenoenzymes to have vastly different kinetic rates than their sulfur analogues. Although formate dehydrogenase is not a heme protein, replacing its selenocysteine with plain cysteine results in a 311-fold reduced activity:⁽¹⁵⁾

Since tRNA and ribosomes cannot differentiate between selenoamino acids and those sulfur-based, some cyclooxygenase-2 must be occasionally-formed with selenomethionine-131. This would of course occur much more frequently upon supplementation, and could potentially lead to reduced heme binding and/or differential catalytic activities. This would of course happen over the course of a few days, and could perhaps explain the long-term reductions of prostaglandin E₂ observed by Baines.
Thomas Baine's doctoral dissertation goes a long way towards understanding the enigmatic effects of selenium on cancer cells.⁽⁶⁾ While aiming to elucidate the primary mechanism of organic selenium species, Doctor Baines had analyzed the effects of selenomethionine and methylselenocysteine on polyamines and prostaglandin E₂. As reliable cancer cell proliferents: these two molecules seem a logical focus and Dr. Baines had previously been familiar with selenomethionine's polyamine inhibition; after all, he had co-authored the Redman article (not that Redman article, the other Redman article).⁽⁷⁾ He had given rats low parts per million doses in their food―
――――――――――――――――――――――――――――――――――――――――――――――――――
It is important to give selenomethionine in food and not water, or you could end-up with results like this:⁽⁸⁾
Doctor Niall Corcoran, a urologist from Australia, had given eight groups or rats eight different selenium species in their drinking water. Upon seeing the results, he trumpets 'inorganic selenium' (sodium selenide) as being the most effective selenium species. What Doctor Corcoran failed to consider, however, is the very low solubility of selenomethionine:⁽⁹⁾
'Solubilities: The solubilities of methionine and of selenomethionine in water at 30°C and pH 7.0 are 0.386 M and 0.108 M, respectively. The fact that selenomethionine is only about one-third as soluble as methionine suggests that the side chain of selenomethionine is more hydrophobic than that of methionine.' ―Shepherd
Methionine has a density of 1.34g/cm³ yet selenomethionine weights 49.3% more. Although Corcoran's flask could initially hold 3·ppm of selenomethionine in solution—as reported—it could potentially form a density gradient over time, depending of course on the time passed between mixing and mouse-administration. Accurate solubilities of selenomethionine do not seem to be commonly reported, and even Sigma–Aldrich lazily uses methionine's solubility to represent that of selenomethionine—as seen here and here—despite it being over three times less.⁽⁹⁾ Although the bottom of Corcoran's volumetric flask most certainly had 3·ppm of selenomethionine, and probably much more, the concentration actually poured in the mouses tongue-activated ball-bearing water dispenser could have been much lower. Since all other studies I've read show selenomethionine to be much more active than selenide, I think it's fair to conclude that the results reported by Doctor Niall Corcoran—a urologist from Australia—had been confounded by water solubility considerations.
――――――――――――――――――――――――――――――――――――――――――――――――――
...and had found the expected cancer reductions. In isolated human cells, selenomethionine had caused reduced cyclooxygenase-2 protein levels after six days despite invariable mRNA levels (in HT-29 cells). Prostaglandin E₂ was correspondingly reduced, and these results were so significant that he'd decided to focus on it.
'The decrease in PGE2 levels correlate well with the growth inhibition seen during the time course.' ―Baines
Yet he doesn't explain it, despite a large proportion of his 146-page dissertation being devoted to the 'discussion.' However, cyclooxygenase-2 does have a methionine in it's catalytic domain at position #131,⁽¹⁰⁾ and a six-day lag period is consistent with enzyme turnover & new protein synthesis.
 click to embiggen
click to embiggenAlthough superoxide or oxygen are commonly assumed to be the other substrate besides arachidonate, detailed studies reveal that peroxynitrite is actually responsible.⁽¹²⁾ Peroxynitrite (ONOO⁻) is formed from the spontaneous interaction of superoxide (Ȯ₂⁻) with nitric oxide (ṄO), and is generally considered the most damaging reactive nitrogen species known. Inducible nitric oxide synthase (iNOS) reliably increases prostaglandin formation, and nitric oxide seems to be why. Peroxynitrite is perhaps known best for its tyrosine adduct, yet it has been shown to interact with lysine and methionine.⁽¹³⁾ Lysine-294 sits at the top of cyclooxygenase's heme ring, while (Se)methionine-131 sits on the bottom:⁽¹¹⁾
Cytochrome C peroxidase is a heme protein with a core iron atom axially-ligated on the underside by a thiol, a cysteine in this case.⁽¹⁴⁾ Since demethylated methionine is also a thiol, as homocysteine, I think it's reasonable to assume that cyclooxygenase's heme iron is complexed in the same way. The homocysteine of cyclooxygenase-2 could be situtated in a way analogous to the thiol–Fe²⁺ interaction of cytochrome C peroxidase (left), and could perhaps be better visualized by considering how cobalamin's central cobalt is complexed (right).

Perhaps heme enzymes normally axially-ligated through methionine exhibit a reduced activity when substituted by selenomethionine? This is not a radical proposal, and mutagenic studies have shown selenoenzymes to have vastly different kinetic rates than their sulfur analogues. Although formate dehydrogenase is not a heme protein, replacing its selenocysteine with plain cysteine results in a 311-fold reduced activity:⁽¹⁵⁾
Since tRNA and ribosomes cannot differentiate between selenoamino acids and those sulfur-based, some cyclooxygenase-2 must be occasionally-formed with selenomethionine-131. This would of course occur much more frequently upon supplementation, and could potentially lead to reduced heme binding and/or differential catalytic activities. This would of course happen over the course of a few days, and could perhaps explain the long-term reductions of prostaglandin E₂ observed by Baines.
[1] Redman, C. "Involvement of polyamines in selenomethionine induced apoptosis and mitotic alterations in human tumor cells." Carcinogenesis (1997)
[2] Hirschman, S. "Interaction of spermine and DNA." Biopolymers (1967)
[3] Fiedorow, P. "The influence of polyamines on polymerase chain reaction (PCR)." Acta biochimica Polonica (1997)
[4] Thomas, T. "Polyamine-induced B-DNA to Z-DNA conformational transition of a plasmid DNA with (dG-dC) n insert." Journal of Biological Chemistry (1991)
[5] Antequera, F. "Number of CpG islands and genes in human and mouse." Proceedings of the National Academy of Sciences (1993)
[6] Baines, A. "The mechanism of action of the anticancer effects of selenomethionine on colon cancer." (2001).
[7] Redman, C. "Inhibitory effect of selenomethionine on the growth of three selected human tumor cell lines." Cancer letters (1998)
[8] Corcoran, N. "Inorganic selenium retards progression of experimental hormone refractory prostate cancer." The Journal of urology (2004)
[9] Shepherd, L. "Some chemical and biochemical properties of selenomethionine." Canadian journal of biochemistry (1969)
[10] Smith, W. "Cyclooxygenases: structural, cellular, and molecular biology." Annual review of biochemistry (2000)
[11] Gupta, K. "The 2.0 Å resolution crystal structure of prostaglandin H₂ synthase-1: structural insights into an unusual peroxidase." Journal of molecular biology (2004)
[12] Salvemini, D. "Nitric oxide activates cyclooxygenase enzymes." Proceedings of the National Academy of Sciences (1993)
[13] Tien, M. "Peroxynitrite-mediated modification of proteins at physiological carbon dioxide concentration." Proceedings of the National Academy of Sciences (1999)
[14] Perera, R. "Neutral thiol as a proximal ligand to ferrous heme iron." Proceedings of the National Academy of Sciences (2003)
[15] Axley, M. "Catalytic properties of an Escherichia coli formate dehydrogenase mutant in which sulfur replaces selenium." PNAS (1991)
[2] Hirschman, S. "Interaction of spermine and DNA." Biopolymers (1967)
[3] Fiedorow, P. "The influence of polyamines on polymerase chain reaction (PCR)." Acta biochimica Polonica (1997)
[4] Thomas, T. "Polyamine-induced B-DNA to Z-DNA conformational transition of a plasmid DNA with (dG-dC) n insert." Journal of Biological Chemistry (1991)
[5] Antequera, F. "Number of CpG islands and genes in human and mouse." Proceedings of the National Academy of Sciences (1993)
[6] Baines, A. "The mechanism of action of the anticancer effects of selenomethionine on colon cancer." (2001).
[7] Redman, C. "Inhibitory effect of selenomethionine on the growth of three selected human tumor cell lines." Cancer letters (1998)
[8] Corcoran, N. "Inorganic selenium retards progression of experimental hormone refractory prostate cancer." The Journal of urology (2004)
[9] Shepherd, L. "Some chemical and biochemical properties of selenomethionine." Canadian journal of biochemistry (1969)
[10] Smith, W. "Cyclooxygenases: structural, cellular, and molecular biology." Annual review of biochemistry (2000)
[11] Gupta, K. "The 2.0 Å resolution crystal structure of prostaglandin H₂ synthase-1: structural insights into an unusual peroxidase." Journal of molecular biology (2004)
[12] Salvemini, D. "Nitric oxide activates cyclooxygenase enzymes." Proceedings of the National Academy of Sciences (1993)
[13] Tien, M. "Peroxynitrite-mediated modification of proteins at physiological carbon dioxide concentration." Proceedings of the National Academy of Sciences (1999)
[14] Perera, R. "Neutral thiol as a proximal ligand to ferrous heme iron." Proceedings of the National Academy of Sciences (2003)
[15] Axley, M. "Catalytic properties of an Escherichia coli formate dehydrogenase mutant in which sulfur replaces selenium." PNAS (1991)
Last edited:
