Are you talking about gut absorption, as per Remer?I don't think that the imbalance from KCl is minimal, it's not like MgCl2 that has one part of magnesium for two parts of chloride, but compartment distribution is different in both cases, there can be a significant amount of unpaired K inside the cell and Cl outside.
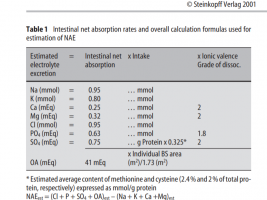
Are you referring to Table 1?
So yes, gut absorption rates are:
Cl 95%
Mg 32%
K 85%
Na 95%
Based on this MgCl2 is a very high acidic load, KCl is lesser, but nonetheless, there is acidic load, but with NaCl, there is no acidic load.