How would a cup or so of "real" homemade gelatin compare to "quick" instant offered by the various well-known brands often mentioned here - sodium content/nutrition content etc. I feel dramatic effects from batches I make at home whereas instant doesn't. Homemade causes me to sleep deeper, instant gives me sluggishness
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
-
By using this site you agree to the terms, rules, and privacy policy.
-
Charlie's Restoration Giveaway #2 (Entire Home EMF Mitigation & Protection Along With Personal Protection) - Click Here To Enter
-
Dear Carnivore Dieters, A Muscle Meat Only Diet is Extremely Healing Because it is a Low "vitamin A" Diet. This is Why it Works so Well...
Rest the rest of this post by clicking here
-
The Forum is transitioning to a subscription-based membership model - Click Here To Read
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What's Better - Real Bone Broth Gelatin Or Powdered/Instant
- Thread starter DMF
- Start date
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
I had the same experience as you did, more so collagen hydrolysate (by Great Lakes Gelatin company) made me feel worse. I did not get any reactions to it like upset stomach, but overtime my hairs lost some shine and i did not see all the good things i experienced with bone broth, more so i felt kind of more tired with it.How would a cup or so of "real" homemade gelatin compare to "quick" instant offered by the various well-known brands often mentioned here - sodium content/nutrition content etc. I feel dramatic effects from batches I make at home whereas instant doesn't. Homemade causes me to sleep deeper, instant gives me sluggishness
I can't say much about their gelatin, because i use it only in marshmallows and alike once a week no more.
I dropped collagen hydrolysate after 4-5 months and got back to making bone broth, and i see (hairs,nails) and feel the difference.
It also tastes amazing and is so nourishing especially during winter months.
raypeatclips
Member
- Joined
- Jul 8, 2016
- Messages
- 2,555
Food sources are always more complete and beneficial. Peat has said before "coffee is more than just caffeine" and he has said "liver is more than just vitamin a" I believe. In the same regard I think, following the trend, and I don't mean to speak for Peat, he might say "bone broths are more than just gelatin"
Dave Clark
Member
- Joined
- Jun 2, 2017
- Messages
- 2,001
Yes, perhaps the chondroitins, etc. that were spoken about in another thread.Food sources are always more complete and beneficial. Peat has said before "coffee is more than just caffeine" and he has said "liver is more than just vitamin a" I believe. In the same regard I think, following the trend, and I don't mean to speak for Peat, he might say "bone broths are more than just gelatin"
ecstatichamster
Member
- Joined
- Nov 21, 2015
- Messages
- 10,521
i've had great success with collagen and so have people I've recommended it to.
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
Here is Great Lakes Beef Gelatin vs Beef Collagen Hydrolysate
comparison - mine, data from Great Lakes
http://greatlakesgelatin.com/business/GelatinSpecs_As_of_March2017.pdf
http://greatlakesgelatin.com/business/GelatinSpecs_Hydro_as_of_2014.pdf
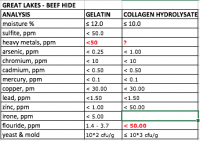
It's weird they skipped Heavy metals total for hydrolysate, or is it to high to display? Because Heavy metals for Gelatin are pretty high.
Fluoride concentration is disturbing as well.
Protein for Collagen Hydrolysate:

Protein content for beef bone broth
from Bone Broth Micro nutrient Panels

How is that: 2 tbsp dry 12 g protein vs 8 ounces watery broth 8 g?
My broth looks like that (petty thick):

And a while ago i found a thread
Collagen Hydrolysate Vs. Gelatin
And do you think hydrolization process may introduce extra heavy metals and what not to the final product?
comparison - mine, data from Great Lakes
http://greatlakesgelatin.com/business/GelatinSpecs_As_of_March2017.pdf
http://greatlakesgelatin.com/business/GelatinSpecs_Hydro_as_of_2014.pdf

It's weird they skipped Heavy metals total for hydrolysate, or is it to high to display? Because Heavy metals for Gelatin are pretty high.
Fluoride concentration is disturbing as well.
Protein for Collagen Hydrolysate:

Protein content for beef bone broth
from Bone Broth Micro nutrient Panels
How is that: 2 tbsp dry 12 g protein vs 8 ounces watery broth 8 g?
My broth looks like that (petty thick):

And a while ago i found a thread
Collagen Hydrolysate Vs. Gelatin
Hey @Travis is it really the case? Or that other Travis is spreading misinformation? :)I was using protein powders there for a quite a while, and there is a huge difference between between a hydrolyzed protein and otherwise.
A protein can be a long chain of amino acids. Hydrolyzing can break the protein down into just several aminos, making a peptide of, say, three aminos. The length and combination of the aminos in the peptide have a large bearing on the bioactivity of the peptide. To illustrate: free aminos absorb much faster than undenatured proteins. But peptides absorb even faster than aminos. So there is a big difference in the original form of the protein, particularly with hydrolysation. Additionally, a protein can be hydrolyzed to varying lengths, they aren't all a 3-amino peptide, some are longer: di-peptides, tri-peptides. Each behaving differently in the process of absorption and subsequent biological effects in the bloodstream. Lactoferrin, for example, is a full protein that, when absorbed as is, undigested, has many immune-boosting effects in the body. So proteins are absorbed as full proteins, and at every stage down to free aminos.
And do you think hydrolization process may introduce extra heavy metals and what not to the final product?
Kunder
Member
- Joined
- Jan 6, 2018
- Messages
- 141
Holy crap, only 8 grams of protein in 227 grams of broth, that is shockingly low. Admitedly it has a high water content for sure, but one would expect more of the good stuff.
By the way, that broth you're showing looks preety murky and greasy, almost like coconut oil. Broth should be almost translucent, and have minimum fat.
By the way, that broth you're showing looks preety murky and greasy, almost like coconut oil. Broth should be almost translucent, and have minimum fat.
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
Here is Great Lakes Beef Gelatin vs Beef Collagen Hydrolysate
comparison - mine, data from Great Lakes
This seems to be a more‐or‐less accurate statement, but the part about peptides absorbing faster than free amino acids is a bit surprising. I don't know if that is true, but I can see it happening because free amino acids will have a larger charge to mass ratio; this could make them more polar and keep them more in the water phase or ionically attracted to other food components.http://greatlakesgelatin.com/business/GelatinSpecs_As_of_March2017.pdf
http://greatlakesgelatin.com/business/GelatinSpecs_Hydro_as_of_2014.pdf
View attachment 7972
It's weird they skipped Heavy metals total for hydrolysate, or is it to high to display? Because Heavy metals for Gelatin are pretty high.
Fluoride concentration is disturbing as well.
Protein for Collagen Hydrolysate:
View attachment 7973
Protein content for beef bone broth
from Bone Broth Micro nutrient Panels
View attachment 7974
How is that: 2 tbsp dry 12 g protein vs 8 ounces watery broth 8 g?
My broth looks like that (petty thick):
View attachment 7975
And a while ago i found a thread
Collagen Hydrolysate Vs. Gelatin
Hey @Travis is it really the case? Or that other Travis is spreading misinformation? :)
And do you think hydrolization process may introduce extra heavy metals and what not to the final product?
When the peptide bond is broken, by either an enzyme of a strong acid, one H₂O molecule is added: a OH⁻ group must go on the C‐end of the amino acid, and a H⁺ or two on the N‐end. The term 'hydrolyzed' is often used by chemists, but other words can also rightly be used and can be more intuitive (i.e. cleaved). Proteolysis is sometimes used as a verb to describe this event, and different enzymes prefferentially cleave peptide bonds at different amino acids ⟶ leading to different fragments.
We'd have to look into the extent of hydrolysis to get an idea of the peptide length. You'd almost think the length would follow a normal distribution (curve), centered around ~ten‐peptide fragments. But since the length of hydrolysis determines the length of the peptides, you'd be certain that it would be highly variable between product types—and perhaps even between manufacturers of the same product.
Since all that are needed are enzymes, you wouldn't expect heavy metals. However! as mentioned previously, strong acids also hydrolyze proteins and could theoretically be used for hydrolysis. If so, you could perhaps anticipate metal contamination if metal reaction vessels are used. Of course in laboratory settings all vessels would all be glass, but large‐scale industrial processes sometimes use metallic vats.
Last edited:
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
R u serious? 8 g of protein in 1 cup of liquid is low? It’s like milk at 1g per oz. And it’s pretty good considering the amino acids profine of such protein. I would say 12g of protein per dry mass (less than 10% moister) is not that high actually.Holy crap, only 8 grams of protein in 227 grams of broth, that is shockingly low. Admitedly it has a high water content for sure, but one would expect more of the good stuff.
Kunder
Member
- Joined
- Jan 6, 2018
- Messages
- 141
Yes except milk is liquid and this is a solid mass. And milk is exactly 3.5 grams of protein per 100 grams, which would make total milk protein content identical to this broth. And that is quite low for a solid food.
By the way i dont understand how you can function mixing the antiquated ounces with grams in one sentence.
By the way i dont understand how you can function mixing the antiquated ounces with grams in one sentence.
Amazoniac
Member
Guru, thanks for collecting those!Here is Great Lakes Beef Gelatin vs Beef Collagen Hydrolysate
comparison - mine, data from Great Lakes
http://greatlakesgelatin.com/business/GelatinSpecs_As_of_March2017.pdf
http://greatlakesgelatin.com/business/GelatinSpecs_Hydro_as_of_2014.pdf
View attachment 7972
It's weird they skipped Heavy metals total for hydrolysate, or is it to high to display? Because Heavy metals for Gelatin are pretty high.
Fluoride concentration is disturbing as well.
Protein for Collagen Hydrolysate:
View attachment 7973
Protein content for beef bone broth
from Bone Broth Micro nutrient Panels
View attachment 7974
How is that: 2 tbsp dry 12 g protein vs 8 ounces watery broth 8 g?
My broth looks like that (petty thick):
View attachment 7975
And a while ago i found a thread
Collagen Hydrolysate Vs. Gelatin
Hey @Travis is it really the case? Or that other Travis is spreading misinformation? :)
And do you think hydrolization process may introduce extra heavy metals and what not to the final product?
It contains quite a lot of lead and other contaminants indeed.
The calculation can be simplified:
1ppm = 10^(-6). However!
 To convert grams to mcg you have to multiply by.. 10^6. So you can just take their suggested serving 12g and multiply by the respective proportion of the contaminant and you end up with the mcg value.
To convert grams to mcg you have to multiply by.. 10^6. So you can just take their suggested serving 12g and multiply by the respective proportion of the contaminant and you end up with the mcg value.Ex. 12*1.5 (lead) = 18mcg
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1475014/pdf/envhper00496-0066.pdf#page=4
Last edited:
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
You probably are not from US.Yes except milk is liquid and this is a solid mass. And milk is exactly 3.5 grams of protein per 100 grams, which would make total milk protein content identical to this broth. And that is quite low for a solid food.
By the way i dont understand how you can function mixing the antiquated ounces with grams in one sentence.
1 cup = 8 oz fluid it may be any amount of grams, depending on the substance.
Amagine a tea cup: fill it up with sugar, fill another with OJ, fill another with cream, fill another with mercury (just kidding, do not do it) and so on, they all will be 1 cup (tea cup) but different by weight in grams (the one with the mercury will be the heaviest about 1 lb per 8 oz)
Broth looks solid and is solid in the fridge, because of the amino acids quilities it contains, at room temperature its mostly liquid, warmed up its liquid like sipping tea.
Last edited:
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
Thank you, as always.This seems to be a more‐or‐less accurate statement, but the part about peptides absorbing faster than free amino acids is a bit surprising. I don't know if that is true, but I can see it happening because free amino acids will have a larger charge to mass ratio; this could make them more polar and keep them more in the water phase or ionically attracted to other food components.
When the peptide bond is broken, by either an enzyme of a strong acid, one H₂O molecule is added: a OH⁻ group must go on the C‐end of the amino acid, and a H⁺ or two on the N‐end. The term 'hydrolyzed' is often used by chemists, but other words can also rightly be used and can be more intuitive (i.e. cleaved). Proteolysis is sometimes used as a verb to describe this event, and different enzymes prefferentially cleave peptide bonds at different amino acids ⟶ leading to different fragments.
We'd have to look into the extent of hydrolysis to get an idea of the peptide length. You'd almost think the length would follow a normal distribution (curve), centered around ~ten‐peptide fragments. But since the length of hydrolysis determines the length of the peptides, you'd be certain that it would be highly variable between product types—and perhaps even between manufacturers of the same product.
Since all that are needed are enzymes, you wouldn't expect heavy metals. However! as mentioned previously, strong acids also hydrolyze proteins and could theoretically be used for hydrolysis. If so, you could perhaps anticipate metal contamination if metal reaction vessels are used. Of course in laboratory settings all vessels would all be glass, but large‐scale industrial processes sometimes use metallic vats.
Any hints on why flouride increases several fold after hydrolysis?
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
Yet to see a guy who complains about antique ounces ordering a pint of beer in places all over the world.antiquated ounces
I guess context is everything indeed LOL
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
You can spot a person who never made bone broth himself at first sight.By the way, that broth you're showing looks preety murky and greasy, almost like coconut oil. Broth should be almost translucent, and have minimum fat.
The broth pictured would be considered pretty translucent for the broth, it also has almost zero fat.
Here is how (specially for you @Kunder cause you obviously have no idea):

after broth is cooled in the fridge all the fat is on top

you can remove it using a spoon

and wipe with paper towel at the end to get rid of any fat left

the little white sediment on top is not fat it's some kind of mineral sediment
The fat in this particular batch is semi solid (after cooling) because it was made out of beef feet cut alongside the bones

and probably contained more mono-unsaturted fats from the bone marrow.
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
If it can be found in collagen, I think you would have to assume that this comes from the cow bones.Thank you, as always.
Any hints on why flouride increases several fold after hydrolysis?
Although flourine gas (F₂) is highly reactive, the fluoride ion (F⁻) is much less so. However! we all know the fluoride ion (F⁻) is still dangerous and should be kept out of food. I wonder if this could add to a protein somehow, perhaps on hydroxyl groups.. .
I wonder if any of these funky fluorinated amino acids can be found in hydrolyzed gelatin. The fluoride ion (F⁻) can also ionically bind the amino groups (–NH₃⁺) of amino acids, so any fluoride released from cow bones during acid hydrolysis could be expected to bind to the peptides in two ways. Although phosphate, calcium, and hydroxide make up the majority of bone, I don't think any of these would bind proteins as strongly as F⁻ (with the exception being O‐phosphorylated tyrosine, serine, and threonine) and would be safe even if they had.
Since fluoride is found in hydrolyzed collagen, I'm getting the impression that acids are used to separate connective tissue from cow bones during routine production; I am also getting the impression that any residual F⁻ was one time fluoroapatite crystals of cow bone.
Last edited:
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
I find the matter-of-fact tone of this article's title somewhat amusing:
Evans, Robert John. "Fluorine storage in cattle bones." Journal of Dairy Science (1938)
Last edited:
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
It was the first thing that came to mind, but:If it can be found in collagen, I think you would have to assume that this comes from the cow bones.
Although flourine gas (F₂) is highly reactive, the fluoride ion (F⁻) is much less so. However! we all know the fluoride ion (F⁻) is still dangerous and should be kept out of food. I wonder if this could add to a protein somehow, perhaps on hydroxyl groups.. .
Fluorotyrosine: a fluoridated amino acid!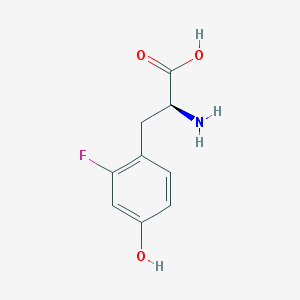
I wonder if any of these funky fluorinated amino acids can be found in hydrolyzed gelatin. The fluoride ion (F⁻) can also ionically bind the amino groups (–NH₃⁺) of amino acids, so any fluoride released from cow bones during acid hydrolysis could be expected to bind to the peptides in two ways. Although phosphate, calcium, and hydroxide make up the majority of bone, I don't think any of these would bind proteins as strongly as F⁻ (with the exception being O‐phosphorylated tyrosine, serine, and threonine) and would be safe even if they had.
Since fluoride is found in hydrolyzed collagen, I'm getting the impression that acids are used to separate connective tissue from cow bones during routine production; I am also getting the impression that any residual F⁻ was one time fluoroapatite crystals of cow bone.
Collagen hydrolysante is made out of gelatin, not directly from the hides (but even if it was the raw material are the beef hides). And gelatin is made out of hides, not bones, and hides are separated manually at the slaughter facilities.
Do you have any other thoughts?
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
@Travis this source describes the process but lacks the details:
Process for Production of Hydrolysed Collagen from Agriculture Resources: Potential for Further Development
Process for Production of Hydrolysed Collagen from Agriculture Resources: Potential for Further Development
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
David Baziwane as late as 2003 indicates the provenance of commercial gelatin to be:It was the first thing that came to mind, but:
Collagen hydrolysante is made out of gelatin, not directly from the hides (but even if it was the raw material are the beef hides). And gelatin is made out of hides, not bones, and hides are separated manually at the slaughter facilities.
Do you have any other thoughts?
'Gelatin is usually derived from sources of collagen that are available both in quantity and at a reasonable price for the manufacturer. Commercially viable sources include mainly demineralized cattle bone (ossein) and bovine and pig skin.' ―Baziwane
Which is hydrolyzed either by an acid or an alkaline solution:
'Commonly used temperatures are 55°C, 60°C, 70°C, and 80–90°C from the first to the final extractions, respectively. There are basically two processes by which collagen is hydrolyzed into gelatin:
i. The acid process, as described in detail by Reich et al. (1992), is mainly used with pigskin, marine fish skin, and sometimes bone raw materials. Here, collagen is acidified to about pH 4 and then heated stepwise from 50°C to boiling point to denature and solubilize the collagen into type A gelatin.
ii. The alkali process is mainly used on bovine hide (Cole and Roberts, 1996). Before extraction, collagen is subjected to a caustic soda or liming process, which quickly hydrolyzes the asparagines and glutamine side chains to glutamic and aspartic acid (Veis, 1964), with the result that gelatin has a traditional isoionic point between four and five.
ii. The alkali process is mainly used on bovine hide (Cole and Roberts, 1996). Before extraction, collagen is subjected to a caustic soda or liming process, which quickly hydrolyzes the asparagines and glutamine side chains to glutamic and aspartic acid (Veis, 1964), with the result that gelatin has a traditional isoionic point between four and five.
Step 2: The extracted gelatin solution is then filtered through diatomaceous earth to remove suspended insolubles such as lipids or unhydrolyzed collagen fibers. The gelatin is then further purified through ion exchange or ultrafiltration columns, which remove inorganic salts and also adjusts the pH to a range of 5.0–5.8 that is suitable for sale.
Step 3: The final stage is dehydration (mainly through spray drying), sterilization, and drying. These three unit processes are performed as quickly as possible to minimize the loss of physical–chemical properties of the resultant gelatin. The obtained product is then subjected to qualitative analysis tests, namely, bloom strength, viscosity, composition, and microbiological tests.' ―Baziwane
Step 3: The final stage is dehydration (mainly through spray drying), sterilization, and drying. These three unit processes are performed as quickly as possible to minimize the loss of physical–chemical properties of the resultant gelatin. The obtained product is then subjected to qualitative analysis tests, namely, bloom strength, viscosity, composition, and microbiological tests.' ―Baziwane
Of course acid hydrolysis of bone would lead to most fluoride and toxic metals: the F⁻ of course coming from the bone and perhaps some metal ions from the tank. I think it would be more economical to hydrolzye the gelatin further at this point; commercial 'hydrolyzed' gelatin simply being allowed to react further, perhaps even with an additional enzymatic step. I think we'd have to look at patents to get a better idea . . . unless you've heard anything directly from the producer?
Baziwane, David. "Gelatin: the paramount food additive." Food Reviews International (2003)
EMF Mitigation - Flush Niacin - Big 5 Minerals
Similar threads
- Replies
- 102
- Views
- 31K
- Replies
- 311
- Views
- 31K
- Replies
- 360
- Views
- 77K
- Replies
- 3
- Views
- 9K
- Replies
- 4
- Views
- 3K
- Replies
- 179
- Views
- 47K
- Replies
- 8
- Views
- 9K
- Replies
- 21
- Views
- 14K
- Replies
- 194
- Views
- 18K
- Replies
- 6
- Views
- 5K
- Replies
- 6
- Views
- 2K
- Replies
- 9
- Views
- 8K
