I would guess that it wouldn't because it would be immobolized. And without any water, it would have no way of diffusing towards the iron. All fruits have low amounts of iron. Get the powder wet and the vitamin C might then find something to react with, somewhere.
But we don't need to get too theoretical, as this has been tested:
Santos, P. H. S., and M. A. Silva. "Retention of vitamin C in drying processes of fruits and vegetables—A review." Drying Technology 26.12 (2008): 1421-1437.
Fruit powder in a bag should mitigate all of these factors, besides temperature.
Be modest now Dr. Santos. He's assuring the readers that he knows calculus. Wait until you see the Thermal Death Time (TDT) integral equation...
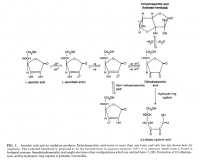
There is a paradoxical effect that doesn't really matter to our topic, but it's interesting nonetheless. When drying, the ascorbic acid oxidation rate actually increases at first because the reactants become more concentrated with less water. Oxidation reaches its peak around 70% water. But after that it drops rapidly. This was done in tomatoes. Don't try to model the oxidation kinetics of tomatoes without a mathematician at hand. Only lychees can be modeled by first-order kinetics. He get's around to explaining the dilemma: If water content increases oxidation, then why doesn't pineapple come fully-oxidized at the store? Apparently fruit has internal structure that keeps things separated. Albert Szent-Györgyi talks about this when he talks about bruising. He's written an entire article on this:
Vitamin C is also a quinone.
Szent-Györgyi, Albert. "Steric, electronic and integrative factors in enzymic regulation." Advances in enzyme regulation 7 (1969): 5-11.Hidden on the bottom of this paper is a table showing references to other articles arranged by fruit. There were two cited for acreola:
Marques, Luanda G., Ana M. Silveira, and José T. Freire. "Freeze-drying characteristics of tropical fruits." Drying technology 24.4 (2006): 457-463.
View attachment 6503Click to embiggen
An insignificant reduction upon freeze-drying the Barbados cherry. The ascorbic acid content was determined using dichlorophenolindophenol titration. This is a redox technique so would be expected to measure full-reduced vitamin C only, the only form with spare electrons to donate to the titrant. The other common technique—ultraviolet absorption—would probably be less accurate in differentiating between the two forms.
Marques, Luanda G., Maria C. Ferreira, and José T. Freire. "Freeze-drying of acerola (Malpighia glabra L.)." Chemical Engineering and Processing: Process Intensification 46.5 (2007): 451-457.
So the Barbados cherry and and acreola berry are synonymous.
View attachment 6504
More losses here, but there is still quite a bit of vitamin C remaining. This is ahighmega-vitamin berry!
You could do it, but it would be somewhat expensive. You would need a way to accurately measure the volume of dichlorophenolindophenol solution delivered the precise mass of the acreola powder to be determined, down to the microgram. A glass burette and an accurate scale would be necessary.
This is the method used Dr. Luanda G Marques:
Harris, Leslie J., and Mamie Olliver. "Vitamin methods: The reliability of the method for estimating vitamin C by titration against 2: 6-dichlorophenolindophenol. 1. Control tests with plant tissues." Biochemical Journal 36.1-2 (1942): 155.
Thank you so much for taking the time to write this down Travis.
Found something else about the stability of ascorbic acid in freeze dried fruit:
Vitamin C Content of Freeze-Dried Tropical FruitsThe stability of ascorbic acid in freeze-dried acerola was studied by Leme et al. [16]. The authors verified an average loss of vitamin C of 5.0% after drying and a loss of 1.0% during storage for 4-9 months at ambient temperature.
Luanda G. Marques, Manoel M. Prado; José T. Freire
The study referenced is in Portuguese though unfortunately.
But it really looks like freeze dried fruit powder could be a viable option for a vitamin c supplement.
Last edited: