magnesiumania
Member
- Joined
- Jul 17, 2018
- Messages
- 607
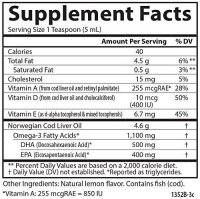
You should probably get some magnesium with such a high dose
Good idea. Magnesium is required for every step in D metabolism. For the convertion to storage form in the liver and to active form in the kidney.
As vit A and D operate very closly you may throw off the A to D balance in the liver.... I would just take cod liver oil for the optimal balance of A to D. (retinol is important for the D receptors to work at all)