hiconscience
Member
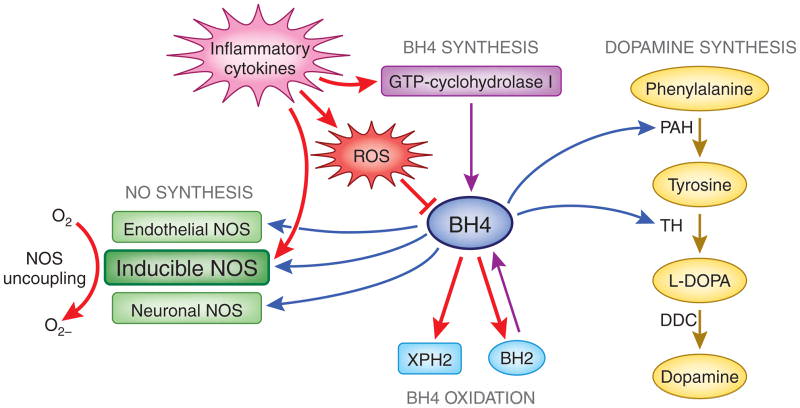
so I have began reading up on BH4 and it is extremely interesting to me. I am wondering what others have uncovered and if they have used BH4 supplementally. it helps create dopamine out of tyrosine lowers free tryptophan. Exogenous estrogen lower BH4... I am also wondering it's effects on growth and the pituitary. Since it helps make dopamine I would think it would lower prolactin formation in the pituitary. also wondering it's effects on liver since it is a B vitamin technically I would think it would be positive.
any input or what to expect from supplementation would be much appreciated!
CPC #9: HASHIMOTO's AND MELASMA: GATEWAY DISEASES - Dr. Jack Kruse
-Holly
any input or what to expect from supplementation would be much appreciated!
CPC #9: HASHIMOTO's AND MELASMA: GATEWAY DISEASES - Dr. Jack Kruse
-Holly