Yes, thank you. My father has what he tells me is a slow growing form and the doctor said if he did nothing (after refusing surgery) that he had about 15 years to live and would most likely die of old age with prostate cancer rather than dying from prostate cancer-FWIW. He now refuses further biopsies and only monitors his PSA every 6 months which has been holding steady at around 4-5 for years. Anecdotally his own father died quite suddenly at around 90 after a long and healthy life, drank apple cider vinegar (heinz) with honey daily for decades and never ever went to doctors. That's why I think something like this would possibly be a good fit for my dad. He has been taking some supplements based on information I discussed with haidut on the forum years ago but prefers to avoid hormones. He doesn't do everything I suggest but he is open-minded.Also would like this post to be seen by @Blossom, when thinking of giving it to your father. We don't want to wake any sleeping giants! Thinking things out clearly, is a must
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
-
By using this site you agree to the terms, rules, and privacy policy.
-
Charlie's Restoration Giveaway #2 (Entire Home EMF Mitigation & Protection Along With Personal Protection) - Click Here To Enter
-
Dear Carnivore Dieters, A Muscle Meat Only Diet is Extremely Healing Because it is a Low "vitamin A" Diet. This is Why it Works so Well...
Rest the rest of this post by clicking here
-
The Forum is transitioning to a subscription-based membership model - Click Here To Read
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Must Read, Killing Cancer Cells Using Electric Potential, DMSO, Methylene Blue
- Thread starter TreasureVibe
- Start date
OP
TreasureVibe
Member
- Joined
- Jul 3, 2016
- Messages
- 1,941
Yes, thank you. My father has what he tells me is a slow growing form and the doctor said if he did nothing (after refusing surgery) that he had about 15 years to live and would most likely die of old age with prostate cancer rather than dying from prostate cancer-FWIW. He now refuses further biopsies and only monitors his PSA every 6 months which has been holding steady at around 4-5 for years. Anecdotally his own father died quite suddenly at around 90 after a long and healthy life, drank apple cider vinegar (heinz) with honey daily for decades and never ever went to doctors. That's why I think something like this would possibly be a good fit for my dad. He has been taking some supplements based on information I discussed with haidut on the forum years ago but prefers to avoid hormones. He doesn't do everything I suggest but he is open-minded.
Yeah, I can't vouch for the safety of this method, nor can I guarantee that it will work.. I would feel enormously bad if this treatment somehow made things worse in a person, so that is why I'm trying to figure out all angles to see if there is truly no "catch". Honey is a great anti-pathogen, I think. See: Honey: its medicinal property and antibacterial activity although Manuka honey seems to be in the spotlights, it could go for all honey.
With methylene blue to back up the electric effect of sodium/potassium bicarbonate + ACV, I think you're safer in terms of aggrevation through electricity than only sodium/potassium bicarbonate + ACV. Might be wrong, but I like to play it on safe. It should really be deepened more.
So far I would use it as an adjuvant in a treatment for cancer, not just as a primary treatment on its own. And I would always use methylene blue along with the sodium/potassium bicarbonate + ACV.
I'll probably wait until I see him in person to talk to him about all of this stuff. He lives out of state and doesn't do the internet/computers so by that point I'll have it all organized enough to present for him. Don't worry though @TreasureVibe. I don't expect you to have the cure for cancer after all! I appreciate the conversation though and information but ultimately we are all responsible for ourselves and our choices. Life is a risk and even if we think we are doing everything right sometimes things don't work out the way we planned. I would never blame you or anyone if he suddenly took a turn for the worse. I'm honestly surprised with the stress of my mom dying that he is holding up so well as it is.Yeah, I can't vouch for the safety of this method, nor can I guarantee that it will work.. I would feel enormously bad if this treatment somehow made things worse in a person, so that is why I'm trying to figure out all angles to see if there is truly no "catch". Honey is a great anti-pathogen, I think. See: Honey: its medicinal property and antibacterial activity although Manuka honey seems to be in the spotlights, it could go for all honey.
With methylene blue to back up the electric effect of sodium/potassium bicarbonate + ACV, I think you're safer in terms of aggrevation through electricity than only sodium/potassium bicarbonate + ACV. Might be wrong, but I like to play it on safe. It should really be deepened more.
So far I would use it as an adjuvant in a treatment for cancer, not just as a primary treatment on its own.
OP
TreasureVibe
Member
- Joined
- Jul 3, 2016
- Messages
- 1,941
Thank you for the comforting words Blossom. I'm sorry for the loss of your mom, it's good to hear that your dad is holding up so well despite it. I wish him well. You're right in that everyone is ultimately responsible for his or her self. And that life is indeed, a risk. And indeed, things don't always go as planned.. That is why we must think things out as much as we can. Life's an adventure or a journey you might say!I'll probably wait until I see him in person to talk to him about all of this stuff. He lives out of state and doesn't do the internet/computers so by that point I'll have it all organized enough to present for him. Don't worry though @TreasureVibe. I don't expect you to have the cure for cancer after all! I appreciate the conversation though and information but ultimately we are all responsible for ourselves and our choices. Life is a risk and even if we think we are doing everything right sometimes things don't work out the way we planned. I would never blame you or anyone if he suddenly took a turn for the worse. I'm honestly surprised with the stress of my mom dying that he is holding up so well as it is.
What is striking, is that the lactic acid environment of the extracellular matrix is actually anti-pathogenic through the acidity. There's studies confirming an anti-pathogenic effect for lactic acid. You would almost think that the tumor-pathogens would like to wipe out any new partygoers trying to join the party, so they keep all the fun to themselves. That, besides the usual functions of lactic acid in cancer cell metabolism and tumor growth. A sense of competition in a tumor is not unfamiliar, as Sloane in his research pointed out that the small amounts of angiogenesis inhibitors the tumor secreted were ment to avoid metastasises (competing tumors) to grow.
What @Obi-wan pointed out is not a small detail. The bicarbonate removing the barrier of the lactic acid in the extracellular matrix is really significant. It implies a whole role of its own for bicarbonate in the treatment of cancer. Without the lactic acid barrier, nutrients can go in, like sodium and potassium acetate, which have strong anti-cancer cell effects like electricity as we've discussed. Since the body continually regulates the pH, the best way of having an effect is taking the bicarbonate directly with an anti-cancer agent like sodium acetate and potassium acetate. But this could go for any anti-cancer cell nutrient. If it's taken with bicarbonate, then there's a bigger chance that it will reach the cancer cell. But, the sodium acetate and the potasssium acetate also perhaps aid in making the cancer cell more (or less, idk for sure) permeable for any nutrient to enter, through their alteration of the cancer cell's membrane voltage adjustment. (the membrane potential Vm) The bicarbonate also makes carbon dioxide, which lowers lactate, and this also means less lactic acid in the lactic acid barrier. Hence making sodium bicarbonate + ACV an ideal combination.
http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1033.3113&rep=rep1&type=pdf is a very interesting read. Very technical.
"Membrane potential (Vm), the voltage across the plasma membrane, arises because of the presence of different ion channels/transporters with specific ion selectivity and permeability. Vm is a key biophysical signal in non-excitable cells, modulating important cellular activities, such as proliferation and differentiation. Therefore, the multiplicities of various ion channels/transporters expressed on different cells are finely tuned in order to regulate the Vm. It is well-established that cancer cells possess distinct bioelectrical properties. Notably, electrophysiological analyses in many cancer cell types have revealed a depolarized Vm that favors cell proliferation. Ion channels/transporters control cell volume and migration, and emerging data also suggest that the level of Vm has functional roles in cancer cell migration. In addition, hyperpolarization is necessary for stem cell differentiation. For example, both osteogenesis and adipogenesis are hindered in human mesenchymal stem cells (hMSCs) under depolarizing conditions. Therefore, in the context of cancer, membrane depolarization might be important for the emergence and maintenance of cancer stem cells (CSCs), giving rise to sustained tumor growth. This review aims to provide a broad understanding of the Vm as a bioelectrical signal in cancer cells by examining several key types of ion channels that contribute to its regulation. The mechanisms by which Vm regulates cancer cell proliferation, migration, and differentiation will be discussed. In the long term, Vm might be a valuable clinical marker for tumor detection with prognostic value, and could even be artificially modified in order to inhibit tumor growth and metastasis."
"The presence of various ion channels and transporters at the plasma membrane provides different permeability to distinct ions, such as Na+,K +, Ca 2+, and Cl −. Due to the unequal distribution of these ions, a voltage difference exists between the cytoplasm and the extracellular environment, which is known as the membrane potential (Vm). Vm is expressed relative to the extracellular environment. A cell is depolarized when the Vm is relatively less negative, whereas a hyperpolarized cell possesses a more negative Vm. Vm changes because of alterations in the conductance of one or more types of ion"
CANCER CELLS POSSESS DEPOLARIZED Vm
THIS says it all! We want a hyperpolarized cell which ACV (potassium acetate) and Baking soda will give. Now add a little Methylene Blue to the picture...-200 to -300 mVolts…
And then their is this "The second point and perhaps the most important point is DMSO (dimethyl sulfoxide) turns cancer cells into normal cells. The mechanism is fairly straightforward. Basically DMSO can enter cancer cells, but the DMSO takes WATER OUT from the cells and reduces their sodium content. When this is done, the cells are no longer waterlogged and normal respiration proceeds. Hence cells are no longer fermentative."
A loud cry to @haidut for a potassium acetate DMSO supplement...
I think I just summarized the title of this thread...Booyah!
IMO sodium bicarbonate creates carbon dioxide which eliminates lactate buildup. That's why you are stronger and have more energy in the gym with less soreness afterward. Cancer cells will build a lactate forcefield around itself which carbon dioxide will remove allowing potassium (ACV) into the cell and creating a hyperpolarized cell. Start doing the ACV/BS combo between meals and see how you feel. I just went out and mowed the lawn like it was nothing. You will become the energizer bunny...Remember fermentation causes NADh buildup which causes Pyruvate buildup which causes Lactic acid build up. Normal respiration creates carbon dioxide which does not occur with fermentation. So cancer cells are like the death star with a lactic acid forcefield...once you get rid of the forcefield and change the electrical potential nutrients will flow in like water flows from high to low. You have now changed the terrain of the cancer cell. Somatids no longer pleomorph into their pathogenic end organism which is fungus that loves an alkaline environment...and fermentation...
Sloane: Another big myth that I always hear and in fact I've read this recently is I keep hearing cancer cannot survive in an alkaline environment, total bogus. No it's not even true at all. In fact the internal pH of a cancer cell is more alkaline than a healthy cell. You can look up the studies on Medline and (unclear word) and show that the internal pH is more alkaline. And the reason for that is that the cancer cells do not generate lactic acid like is commonly told they actually generate lactate which is non-acidic. The acidity comes from the hydrogen protons and the cancer cells cannot tolerate the acidity so they export those hydrogen protons to the external matrix and that is what causes the acidity. So they're actually getting rid of the acidity to protect themselves. It's that alkalinity that allows them to survive and thrive. If people really wanna alkalinize all they really have to do is hyperventilate and that causes them to go very alkaline very quick because you're blowing off so much carbon dioxide that it actually will raise your pH and what happens when you hyperventilate you end up passing out. Well the reason for that is that you need the carbon dioxide or carbonic acid technically in order to maintain the blood vessels in an open state. When you go too alkaline it actually constricts the blood vessels causing a decrease of blood flow going to the brain so you end up passing out. And when you pass out you actually your respiration will slow down or stop temporarily and what that does is it builds back up the carbonic acid to dilate the blood vessels to restore blood flow back to the brain.
According to this source, a chemical engineer, organic acids also metabolize to carbon dioxide (and water) in the body. So the acetic acid in apple cider vinegar also has synergistic effects with the bicarbonate in producing carbon dioxide:
Andrew Kurtz, Chemical engineer
Answered Jan 5 2017
Organic acids don’t become alkaline, but they do metabolize to CO2 and H2O, and leave the body in normal ways. This is in stark contrast to inorganic acids (phosphoric which is in colas, hydrochloric, sulfuric, etc.) which push the body in the acidic direction and contribute to bone loss, kidney problems, hypertension, etc.
Source: https://www.quora.com/How-does-apple-cider-vinegar-become-alkaline-when-its-inside-the-body
All in all, this is fascinating material and there's a role for oral bicarbonate on its own in cancer treatment by its capability of removing the lactic acid blockage in the extracellular matrix of a cancer cell. The carbon dioxide produced by bicarbonate when taken, will lower lactate buildup inside the cancer cell which will also turn into lower lactic acid secretion by the cancer cell into the extracellular matrix.
This can explain all the miracle cures reported by people with cancer taking only sodium bicarbonate, and also explain the curing of cancer patients by Dr. Simoncini with sodium bicarbonate. Dr. Simoncini claimed a 50% cure rate in all of his patients, I think.
According to Russian studies from the 80s, the stomach is permeable leading anything that goes through it directly into the bloodstream, and absorbs oxygen in water 40% faster into the blood than the lungs absorb oxygen.
I wonder what the effects of sodium/potassium bicarbonate + antibiotics in cancer would be!
If I am wrong, please someone correct me! I want to make sure I get this thing right.
I have made a drawing to explain to anyone who doesn't understand what I am talking about to understand what I mean. Click the picture to view the full large version:
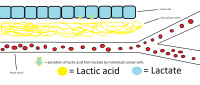
My drawing is based on this picture Extracellular matrix - Wikipedia from this Wikipedia article: Extracellular matrix - Wikipedia
The "Extracellular matrix" is where the ''lactic acid forcefield" resides, making it impossible for nutrients/anti-cancer agents to reach the cancer cells. The nutrients/anti-cancer agents have to come from the blood vessel.
Tumors also have veins from blood vessels leading into them that they grow on their own (this is called angiogenesis) which also possibly contain lactic acid, although I'm not sure about that. I have not included these tumor veins in this picture. If this picture I have made is incorrect, then please correct me.
The red circles inside the blood vessel represent the red blood cells/platelets etc.
Sodium/potassium bicarbonate can remove this lactic acid forcefield temporarily by lowering lactate in the cancer cell (which means less excretion of lactic acid by the cancer cell) and by removing the lactic acid by alkalizing it. When the forcefield is temporarily removed, an anti-cancer agent like sodium acetate or potassium acetate or any anti-cancer agent can reach the cancer cell and possibly enter into it.
Last edited:
OP
TreasureVibe
Member
- Joined
- Jul 3, 2016
- Messages
- 1,941
Bump - for the picture I've made with description. Is this what you've ment, @Obi-wan? (click the picure)
Last edited:
Thanks, she is currently dying (on hospice) but still with us and my dad is caring for her full time. Sorry for the confusion.I'm sorry for the loss of your mom, it's good to hear that your dad is holding up so well despite it.
I really appreciate your detailed contributions on this subject!
OP
TreasureVibe
Member
- Joined
- Jul 3, 2016
- Messages
- 1,941
My apology, I am sorry to hear about your mom being in such a state. Prayers for her. Stay strong for your dad and you and the rest of the family and friends. If advice is required for her condition feel free to inquire.Thanks, she is currently dying (on hospice) but still with us and my dad is caring for her full time. Sorry for the confusion.
I really appreciate your detailed contributions on this subject!
Thank you for your appreciation. I am glad to contribute and help people out, and spark new ideas.
I have made a new rendition of the drawing I've made earlier, which I think is a bit more accurate. The idea is about the same, but the extracellular matrix is actually a membrane that sits next to the cells, according to Basement membrane - Wikipedia. I am uncertain if the basement membrane of the endothelial cells (blood vessel cells) also get lactic acid in them when cancer cells excrete lactic acid, I just added a bit yellow to it and supposed it does but it could not be the case. Lactic acid goes to the liver after all in cancer. See sources beneath picture, which does talk about lactic acid reaching into the endothelial extracellular matrix.*
Click for full large picture:
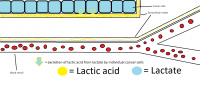
Note the light blue cells represent a cancer tumor.
Which one is right @Obi-wan? I think both give about the same idea, and it is the actual location of the tumor that dictates the surrounding details but all in all I think the second is more accurate.
References:
Will cancer cells be defeated by sodium bicarbonate?
2017
"Chao et al. reported an improved version of the transarterial chemoembolization (TACE) procedure for large hepatocellular carcinoma (HCC), in which 5% sodium bicarbonate was infused into tumors to supplement chemotherapeutic agents. In a small randomized control trial, TACE combined with bicarbonate yielded a 100% objective response rate (ORR), a significant improvement from the 63.6% ORR in the TACE alone group (Chao et al., 2016).
This optimized procedure is termed targeting-intratumoral-lactic-acidosis TACE (TILA-TACE), as the authors expect sodium bicarbonate to interrupt lactic acidosis of tumors. Previously, in vitro studies from the same group suggested that lactic acidosis could effectively protect cancer cells against glucose starvation or deprivation (Wu et al., 2012). The phenomenon of lactic acidosis is characterized with an increase in acidity (acidosis) and a buildup of lactate (lactosis). By directly injecting sodium bicarbonate into tumors, the TILA-TACE procedure is expected not to change the lactate concentration in the tumor microenvironment, but to increase the pH to ameliorate acidosis. Thus, the procedure should be considered as an interruption for acidosis instead of lactic acidosis. However, the in vitro study suggested that acidosis alone, but not lactosis, significantly prolonged the survival time of cancer cells under glucose deprivation (Wu et al., 2012). Since the acidosis is more critical for the survival of tumor cells, it would not be a surprise to see a dramatic effect on tumor apoptosis by simply modulating the pH of the tumor microenvironment with sodium bicarbonate."
Systemic Buffers in Cancer Therapy: The Example of Sodium Bicarbonate; Stupid Idea or Wise Remedy?
2015
"Despite recent therapeutic progress, cancer remains a major cause of death in industrialized countries. As a consequence, alternative treatments attract the attention of a growing number of patients. Among these therapies, the use of sodium bicarbonate to fight cancer has gained considerable interest. According to self-medication reports available on the internet, sodium bicarbonate is viewed by many patients as a simple, costless and efficient anti-cancer agent. Although no clinical study has demonstrated an anti-cancer activity of sodium bicarbonate up to date, emerging experimental reports indicate, that sodium bicarbonate may slow the progression of cancer. Here, we highlight the rationale to use sodium bicarbonate in cancer therapy and further enumerate experimental evidence for its anti-tumoral activity. Finally, we speculate about a future role of sodium bicarbonate in cancer therapy."
"Finally, the effect of tumor acidity on endothelial cells has been reported* but needs further characterization. Nevertheless, it was suggested that acidity would not impair the angiogenic response necessary for tumor growth. In the rat aortic ring model, acidity reduced the angiogenic response but did not block it [33]. In addition, lactic acid might also contribute to angiogenesis by inducing the production of pro-angiogenic factors such as interleukin-8 and bFGF in endothelial cells following its uptake through the lactate transporter MCT1 [34,35]."
Last edited:
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
No, because at vinegar pH most will be acetic acid. For confirmation of this, we can use its pKa and the Henderson–Hasselbalch equation:...but ACV is already an acetate...
pH = pKa + log₁₀([A⁻]/[AH])
And a few constants that are easy-to-find:
pKa = 4.76
pH = 3.4
pH = 3.4
And this equation will give the the relative distribution of molecular species, either: protonated acetic acid (AH), or deprotonated acetate (A⁻):
pH = pKa + log₁₀([A⁻]/[AH])
3.4 = 4.76 + log₁₀([A⁻]/[AH])
3.4 − 4.76 = log₁₀([A⁻]/[AH])
−1. 36 = log₁₀([A⁻]/[AH])
10⁽⁻¹˙³⁶⁾ = [A⁻]/[AH]
.0437 = [A⁻]/[AH]
.0437 × [AH] = [A⁻]
3.4 = 4.76 + log₁₀([A⁻]/[AH])
3.4 − 4.76 = log₁₀([A⁻]/[AH])
−1. 36 = log₁₀([A⁻]/[AH])
10⁽⁻¹˙³⁶⁾ = [A⁻]/[AH]
.0437 = [A⁻]/[AH]
.0437 × [AH] = [A⁻]
For every 100 protonated acetic acid molecules, there are only 4.37 acetate molecules (At pH 3.4).
OP
TreasureVibe
Member
- Joined
- Jul 3, 2016
- Messages
- 1,941
Benzoates are derived from benzoic acid and are more commonly used as food preservatives than the acid. Some people develop allergy-like symptoms when they are exposed to sodium benzoate. When the chemical reacts with vitamin C (ascorbic acid) in drinks under certain conditions, benzene may be produced. Benzene is a carcinogen. A carcinogen is a substance that is capable of causing cancer.now sip Oxidal in water afterward...
Health Effects of Benzoic Acid, Sodium Benzoate, and Benzene
Benzoic acid increases the liver's workload, particularly if consumed with the amino acid glycine, found in protein-rich foods, dietary supplements, and antacids.
Safety Information for Benzoic Acid
Carbonated drinks containing benzoic acid, vitamin C mix can cause cancer, says WHO
Carbonated drinks containing benzoic acid, vitamin C mix can cause cancer, says WHO
Not sure if this is accurate but I thought you should know.
I got a question:
Would generating bicarbonate inside the cancer cell from lactate help for treating cancer? When you oxidize lactate, it will generate bicarbonate.
Bicarbonate therapy in lactic acidosis
INTRODUCTION
Lactic acidosis causes a decrease in serum bicarbonate concentration that is similar in magnitude to the increase in the lactate concentration. Lactate is a metabolizable organic anion that, when oxidized, will generate bicarbonate. Thus, if the stimulus to lactic acid production is eliminated by successful treatment of the underlying disease (eg, restoration of perfusion in a patient with shock), oxidative processes will metabolize the accumulated lactate and regenerate bicarbonate. This will correct the metabolic acidosis and reduce the anion gap.
Source: UpToDate
Last edited:
OP
TreasureVibe
Member
- Joined
- Jul 3, 2016
- Messages
- 1,941
A more simple rendition of what we're speaking of:
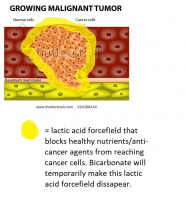
Where I disregard the lactic acid forcefield comprising of the basement membrane and simply show a more simplistic view of what we're dealing with, with cancer.
Does anyone know if a cell can go into a state of fermentation without pathogens being involved? (with state I mean a long term state of primarily fermentation for energy, instead of respiration)

Where I disregard the lactic acid forcefield comprising of the basement membrane and simply show a more simplistic view of what we're dealing with, with cancer.
Does anyone know if a cell can go into a state of fermentation without pathogens being involved? (with state I mean a long term state of primarily fermentation for energy, instead of respiration)
Last edited:
@TreasureVibe, thanks for the prayers for my family.
I was wondering if you've read this article?
Carbonic Anhydrase Inhibitors as Cancer Therapy – Functional Performance Systems (FPS)
I was wondering if you've read this article?
Carbonic Anhydrase Inhibitors as Cancer Therapy – Functional Performance Systems (FPS)
OP
TreasureVibe
Member
- Joined
- Jul 3, 2016
- Messages
- 1,941
Wow good article. Ofcourse no problem!@TreasureVibe, thanks for the prayers for my family.
I was wondering if you've read this article?
Carbonic Anhydrase Inhibitors as Cancer Therapy – Functional Performance Systems (FPS)
Organic acids metabolize into carbon dioxide, ACV contains organic acids. Sodium bicarbonate converts to carbon dioxide in the stomach, I think.
If you were to nebulize sodium bicarbonate, would it convert into carbon dioxide in the lungs? Or is bag breathing a better and more simple option for that..
Btw what people tend to forget is, in this match of "chess" with cancer, there is someone else sitting on the other side of the table. It's pathogens, who are actively piloting/driving the tumor, or so to speak, I think. Even if you can push back the tumor through metabolic means, I think you should not forget that you have to take the pilot out of as well. That means the pathogens.
The chess table is your body's metabolism, and you as your own doctor are sitting at the table, with on the other side of the table as your opponent there's a big roach representing the pathogens gang that runs the cancer tumor for their own liking.
And even afterwards, the immune system must be reinstated so the pathogens can't get back into the driver's seat. "get out, and stay out". The immune system is basically the law system that keeps the criminals from hijacking cells and forming gang hideouts with big sugary-prebiotic metabolism going on.
I wonder what happens if you take sodium/potassium bicarbonate with antibiotics or antivirals.. Bicarbonate opens the gate to the cancer cells by removing the lactic acid barrier.
It's either the bicarbonate or the carbon dioxide that removes the lactic acid barrier, when you take sodium bicarbonate orally. I wonder which one it is.
- Btw - I know this is all off-topic but I think it's still important. Pau D'Arco contains saponins and saponins cause holes in the gut cells membrane, allowing anything that resides in the gut, like pathogens.. to enter freely into the bloodstream. Pau D'Arco is also rich in iron, which pathogens thrive on. So I wouldn't be so keen about Pau D'Arco.. When I took Pau D'Arco I felt pain and sick in my gut.
Garlic also causes holes in the gut lining.
Garlic burns; it literally can burn little holes through the lining of the intestines. Also, it can certainly penetrate the mucus lining and make its way through into the intestinal wall, where it can burn little holes that eventually can lead to perforations, if you eat it too often. And that is the part where they already have been eaten or damaged. So particularly people who have leaky gut, where the toxins already escape from the intestines and move into the blood stream, should be extra careful. This is because garlic should not really be in the blood.
Eating Garlic is Very Toxic for the Body - Ener-Chi Wellness Center
Unsure if it's the DMSO that's in the garlic that's causing this...
Oil of Oregano doesn't contain saponins I think. There is a report though that it also damaged someone's gut lining:
Oregano OIl and damage to the intestinal tract. Help!
Unsure about aspirin either.. With all of it's anti-cancer benefits.
Anything that will make holes in the gut lining will allow endotoxin and pathogens leak into the bloodstream from the insides of the gut, IMO. Causing cancer and overburdening the immune system, so cancer can thrive as well.
That makes me think: Opening the lactic acid barrier with sodium/potassium bicarbonate ALSO causes pathogens to get into the cancer cells. So if one has a poor immune system and/or alot of pathogens in the blood, then it could be a bad idea to use sodium/potassium bicarbonate for cancer.
Last edited:
OP
TreasureVibe
Member
- Joined
- Jul 3, 2016
- Messages
- 1,941
Put 2 big tablespoons of apple cider vinegar in an empty mug. Put half a teaspoon of sodium bicarbonate in it. A big fizzing with foam occured, the foam stayed for a minute or two. Tried smelling the substance while it was fizzing, and a toxic smelling, high-making gas came off it, which made me feel lightheaded upon inhalation. After the fizzing was done, I was interested if the reacted substance was still sour tasting, as the color was still that of the apple cider vinegar. Tried tasting it, then drank it, and it wasn't sour. But it did taste yuck, the taste of sodium bicarbonate with a weird toned down taste. I did put a tip of the teaspoon of baking soda in it after it was done fizzing to see if it would still react, it didn't. So I might've tasted that extra baking soda too.
Anyways, I gulped it down. It was drinkable but didn't taste good imo. (which was expected) I keep getting immune active/metabolic reactions in the chest area I can feel after drinking a combination of ACV and baking soda, wether in water or undiluted. Can't really say for now wether that is good or that is bad, as my hormonal situation, immune system, liver and metabolism on top of this chronic chest infection are currently in very bad shape. But overall it does help take tension away from the painful area.
Interested in making my own potassium acetate capsules and potassium bicarbonate capsules, without fillers. Maybe mix in potassium ascorbate, if it's reacted with fruit derived vitamin C.
Have some methylene blue with it.
I would avoid sodium.. Even if cancer cell's have an affinity for it, I would doubt. Basically taking moderate to high doses of sodium ascorbate w/ bicarb would have the same electrical effect on cancer right? Yet sodium ascorbate (commonly sold as supplement) doesn't sound so attractive as a Peater.. So yeah.
I have potassium bicarbonate capsules here but they contain the soap substance magnesium stearate. Does anyone know if it's safe to react it with ACV and drink it? Will the potassium bicarb and magnesium stearate separate during the reaction? I think I've read on Danny's toxinless.com that magnesium stearate is soapy industrial garbage in a comment section.
Edit: Found out for myself. These potassium bicarbonate capsules by Life Enhancement have atleast a big tip of a teaspoon of magnesium stearate. All of it floated to the top, even after stirring alot. I wouldn't drink this. Need me some pure USP grade potassium bicarbonate and potassium acetate!
Btw - Obi, I really wouldn't be a fond of sodium with cancer, you've said yourself you had increasing symptoms when taking salt in the diet so.. You could be getting tons of sodium in your system through the sodium bicarbonate so.. IDK.
There is more to the therapy than juices and coffee enemas. "Table salt is a poison," says the narration. Our "unrelenting" use of sodium "causes displacement of potassium found naturally in human cells, leaving them vulnerable to attack by disease." For this reason, debated to this day, Dr. Gerson gave patients on his already very-low-sodium, potassium-rich diet still more supplemental potassium in the form of equal parts of potassium gluconate, potassium acetate, and mono potassium phosphate. Flaxseed oil was the preferred fatty acid source, and was to be "raw and cold" and not to be used for a cooking oil. Pancreatin, acidophilus, and vitamin B-3 (niacin) were also provided supplementally.
Source: gersontherapy2
Anyways, I gulped it down. It was drinkable but didn't taste good imo. (which was expected) I keep getting immune active/metabolic reactions in the chest area I can feel after drinking a combination of ACV and baking soda, wether in water or undiluted. Can't really say for now wether that is good or that is bad, as my hormonal situation, immune system, liver and metabolism on top of this chronic chest infection are currently in very bad shape. But overall it does help take tension away from the painful area.
Interested in making my own potassium acetate capsules and potassium bicarbonate capsules, without fillers. Maybe mix in potassium ascorbate, if it's reacted with fruit derived vitamin C.
Have some methylene blue with it.
I would avoid sodium.. Even if cancer cell's have an affinity for it, I would doubt. Basically taking moderate to high doses of sodium ascorbate w/ bicarb would have the same electrical effect on cancer right? Yet sodium ascorbate (commonly sold as supplement) doesn't sound so attractive as a Peater.. So yeah.
I have potassium bicarbonate capsules here but they contain the soap substance magnesium stearate. Does anyone know if it's safe to react it with ACV and drink it? Will the potassium bicarb and magnesium stearate separate during the reaction? I think I've read on Danny's toxinless.com that magnesium stearate is soapy industrial garbage in a comment section.
Edit: Found out for myself. These potassium bicarbonate capsules by Life Enhancement have atleast a big tip of a teaspoon of magnesium stearate. All of it floated to the top, even after stirring alot. I wouldn't drink this. Need me some pure USP grade potassium bicarbonate and potassium acetate!
Btw - Obi, I really wouldn't be a fond of sodium with cancer, you've said yourself you had increasing symptoms when taking salt in the diet so.. You could be getting tons of sodium in your system through the sodium bicarbonate so.. IDK.
There is more to the therapy than juices and coffee enemas. "Table salt is a poison," says the narration. Our "unrelenting" use of sodium "causes displacement of potassium found naturally in human cells, leaving them vulnerable to attack by disease." For this reason, debated to this day, Dr. Gerson gave patients on his already very-low-sodium, potassium-rich diet still more supplemental potassium in the form of equal parts of potassium gluconate, potassium acetate, and mono potassium phosphate. Flaxseed oil was the preferred fatty acid source, and was to be "raw and cold" and not to be used for a cooking oil. Pancreatin, acidophilus, and vitamin B-3 (niacin) were also provided supplementally.
Source: gersontherapy2
Last edited:
Obi-wan
Member
- Joined
- Mar 16, 2017
- Messages
- 1,120
No, because at vinegar pH most will be acetic acid. For confirmation of this, we can use its pKa and the Henderson–Hasselbalch equation:
pH = pKa + log₁₀([A⁻]/[AH])
And a few constants that are easy-to-find:
pKa = 4.76
pH = 3.4
And this equation will give the the relative distribution of molecular species, either: protonated acetic acid (AH), or deprotonated acetate (A⁻):
pH = pKa + log₁₀([A⁻]/[AH])
3.4 = 4.76 + log₁₀([A⁻]/[AH])
3.4 − 4.76 = log₁₀([A⁻]/[AH])
−1. 36 = log₁₀([A⁻]/[AH])
10⁽⁻¹˙³⁶⁾ = [A⁻]/[AH]
.0437 = [A⁻]/[AH]
.0437 × [AH] = [A⁻]
For every 100 protonated acetic acid molecules, there are only 4.37 acetate molecules (At pH 3.4).
I stand corrected Travis you are correct:
Vinegar is a liquid consisting of about 5–20% acetic acid (CH3COOH), water (H2O)
An acetate /ˈæsɪteɪt/ is a salt formed by the combination of acetic acid with an alkaline, earthy, metallic or nonmetallic and other base.
The acetate anion, [CH3COO]−,(or [C2H3O2]−) is one of the carboxylate family. It is the conjugate base of acetic acid. Above a pH of 5.5, acetic acid converts to acetate:[2]
CH3COOH ⇌ CH3COO− + H+
Many acetate salts are ionic, indicated by their tendency to dissolve well in water. A commonly encountered acetate in the home is sodium acetate, a white solid that can be prepared by combining vinegar and sodium bicarbonate ("bicarbonate of soda"):
Sodium acetate, CH3COONa, also abbreviated NaOAc,[8] is the sodium salt of acetic acid
Sodium bicarbonate (IUPAC name: sodium hydrogen carbonate), commonly known as baking soda, is a chemical compound with the formula NaHCO3. It is a salt composed of sodium ions and bicarbonate ions.
Sodium bicarbonate mixed with water can be used as an antacid to treat acid indigestion and heartburn.[27] Its reaction with stomach acid produces salt, water, and carbon dioxide:
Sodium bicarbonate reacts with acetic acid (found in vinegar), producing sodium acetate, water, and carbon dioxide:
Acetate is a common anion in biology. It is mainly utilized by organisms in the form of acetyl coenzyme A.
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism.[1] Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production. Coenzyme A (CoASH or CoA) consists of a β-mercaptoethylamine group linked to the vitamin pantothenic acid through an amide linkage [2] and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol).
CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates through glycolysis and by the breakdown of fatty acids through β-oxidation. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is oxidized to carbon dioxide and water, and the energy released captured in the form of 11 ATP and one GTP per acetyl group.
At high glucose levels, acetyl-CoA is produced through glycolysis.[10] Pyruvate undergoes oxidative decarboxylation in which it loses its carboxyl group (as carbon dioxide) to form acetyl-CoA, giving off 33.5 kJ/mol of energy. The oxidative conversion of pyruvate into acetyl-CoA is referred to as the pyruvate dehydrogenase reaction. It is catalyzed by the pyruvate dehydrogenase complex.
At low glucose levels, the production of acetyl-CoA is linked to β-oxidation of fatty acids. Fatty acids are first converted to acyl-CoA. Acyl-CoA is then degraded in a four-step cycle of dehydrogenation, hydration, oxidation and thiolysis catalyzed by four respective enzymes, namely acyl-CoA dehydrogenase, enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and thiolase. The cycle produces a new acyl-CoA with two fewer carbons and acetyl-CoA as a byproduct.[11]
Acetyl-CoA reacts with oxaloacetate to form citrate, which is then oxidized to CO2 in the cycle
Acetyl-CoA is also the source of the acetyl group incorporated onto certain lysine residues of histone and nonhistone proteins in the posttranslational modification acetylation. This acetylation is catalyzed by acetyltransferases. This acetylation affects cell growth, mitosis, and apoptosis.[18]
- Acetyl-CoA serves as an allosteric regulator of pyruvate dehydrogenase kinase (PDK). It regulates through the ratio of acetyl-CoA versus CoA. Increased concentration of acetyl-CoA activates PDK.[19]
- Acetyl-CoA is also an allosteric activator of pyruvate carboxylase.[
Obi-wan
Member
- Joined
- Mar 16, 2017
- Messages
- 1,120
Per Ray:
Warburg, Koch, and Szent-Gyorgyi had a comprehensive view of biology, in which the aerobic production of lactate, resulting from a respiratory defect, itself was functionally related to the nature of cancer. Carbon Dioxide opposes Lactate.
Sodium bicarbonate reacts with acetic acid (found in vinegar), producing sodium acetate, water, and carbon dioxide:
At high glucose levels, acetyl-CoA is produced through glycolysis.[10] Pyruvate undergoes oxidative decarboxylation in which it loses its carboxyl group (as carbon dioxide)
But what is interesting is At low glucose levels, the production of acetyl-CoA is linked to β-oxidation of fatty acids. No carbon dioxide. Case for NOT limiting glucose which Ray has mentioned many times
Acetyl-CoA reacts with oxaloacetate to form citrate, which is then oxidized to CO2 in the cycle. More Carbon dioxide!
But since cancer is a respiratory defect I think it gobbles up glucose and no Acetyl-CoA is produced and Pyruvate doe not undergoes oxidative decarboxylation so it builds up and converts to Lactate. Acetate would fix this...via CO2...
Now add potassium ascorbate to the picture. Per Ted -As for the ascorbate, which is an alkaline form of vitamin C, it kills them again by their negative millivolts. Ascorbate kills them with the -200 or -100 millivolts depending on the dose. and the potassium itself is toxic to cancer cells
Warburg, Koch, and Szent-Gyorgyi had a comprehensive view of biology, in which the aerobic production of lactate, resulting from a respiratory defect, itself was functionally related to the nature of cancer. Carbon Dioxide opposes Lactate.
Sodium bicarbonate reacts with acetic acid (found in vinegar), producing sodium acetate, water, and carbon dioxide:
At high glucose levels, acetyl-CoA is produced through glycolysis.[10] Pyruvate undergoes oxidative decarboxylation in which it loses its carboxyl group (as carbon dioxide)
But what is interesting is At low glucose levels, the production of acetyl-CoA is linked to β-oxidation of fatty acids. No carbon dioxide. Case for NOT limiting glucose which Ray has mentioned many times
Acetyl-CoA reacts with oxaloacetate to form citrate, which is then oxidized to CO2 in the cycle. More Carbon dioxide!
But since cancer is a respiratory defect I think it gobbles up glucose and no Acetyl-CoA is produced and Pyruvate doe not undergoes oxidative decarboxylation so it builds up and converts to Lactate. Acetate would fix this...via CO2...
Now add potassium ascorbate to the picture. Per Ted -As for the ascorbate, which is an alkaline form of vitamin C, it kills them again by their negative millivolts. Ascorbate kills them with the -200 or -100 millivolts depending on the dose. and the potassium itself is toxic to cancer cells
Last edited:
Obi-wan
Member
- Joined
- Mar 16, 2017
- Messages
- 1,120
Put 2 big tablespoons of apple cider vinegar in an empty mug. Put half a teaspoon of sodium bicarbonate in it. A big fizzing with foam occured, the foam stayed for a minute or two. Tried smelling the substance while it was fizzing, and a toxic smelling, high-making gas came off it, which made me feel lightheaded upon inhalation. After the fizzing was done, I was interested if the reacted substance was still sour tasting, as the color was still that of the apple cider vinegar. Tried tasting it, then drank it, and it wasn't sour. But it did taste yuck, the taste of sodium bicarbonate with a weird toned down taste. I did put a tip of the teaspoon of baking soda in it after it was done fizzing to see if it would still react, it didn't. So I might've tasted that extra baking soda too.
Anyways, I gulped it down. It was drinkable but didn't taste good imo. (which was expected) I keep getting immune active/metabolic reactions in the chest area I can feel after drinking a combination of ACV and baking soda, wether in water or undiluted. Can't really say for now wether that is good or that is bad, as my hormonal situation, immune system, liver and metabolism on top of this chronic chest infection are currently in very bad shape. But overall it does help take tension away from the painful area.
Interested in making my own potassium acetate capsules and potassium bicarbonate capsules, without fillers. Maybe mix in potassium ascorbate, if it's reacted with fruit derived vitamin C.
Have some methylene blue with it.
I would avoid sodium.. Even if cancer cell's have an affinity for it, I would doubt. Basically taking moderate to high doses of sodium ascorbate w/ bicarb would have the same electrical effect on cancer right? Yet sodium ascorbate (commonly sold as supplement) doesn't sound so attractive as a Peater.. So yeah.
I have potassium bicarbonate capsules here but they contain the soap substance magnesium stearate. Does anyone know if it's safe to react it with ACV and drink it? Will the potassium bicarb and magnesium stearate separate during the reaction? I think I've read on Danny's toxinless.com that magnesium stearate is soapy industrial garbage in a comment section.
Edit: Found out for myself. These potassium bicarbonate capsules by Life Enhancement have atleast a big tip of a teaspoon of magnesium stearate. All of it floated to the top, even after stirring alot. I wouldn't drink this. Need me some pure USP grade potassium bicarbonate and potassium acetate!
Btw - Obi, I really wouldn't be a fond of sodium with cancer, you've said yourself you had increasing symptoms when taking salt in the diet so.. You could be getting tons of sodium in your system through the sodium bicarbonate so.. IDK.
There is more to the therapy than juices and coffee enemas. "Table salt is a poison," says the narration. Our "unrelenting" use of sodium "causes displacement of potassium found naturally in human cells, leaving them vulnerable to attack by disease." For this reason, debated to this day, Dr. Gerson gave patients on his already very-low-sodium, potassium-rich diet still more supplemental potassium in the form of equal parts of potassium gluconate, potassium acetate, and mono potassium phosphate. Flaxseed oil was the preferred fatty acid source, and was to be "raw and cold" and not to be used for a cooking oil. Pancreatin, acidophilus, and vitamin B-3 (niacin) were also provided supplementally.
Source: gersontherapy2
I took Flax seed oil and fish oil every day before my PSA hit 13,000. NOT!!!!
"Tried smelling the substance while it was fizzing, and a toxic smelling, high-making gas came off it, which made me feel lightheaded upon inhalation"
- For laboratory use, sodium acetate is inexpensive and usually purchased instead of being synthesized. It is sometimes produced in a laboratory experiment by the reaction of acetic acid, commonly in the 5–8% solution known as vinegar, with sodium carbonate ("washing soda"), sodium bicarbonate ("baking soda"), or sodium hydroxide ("lye", or "caustic soda"). Any of these reactions produce sodium acetate and water. When a sodium and carbonate ion-containing compound is used as the reactant, the carbonate anion from sodium bicarbonate or carbonate, reacts with hydrogen from the carboxyl group (-COOH) in acetic acid, forming carbonic acid. Carbonic acid readily decomposes under normal conditions into gaseous carbon dioxide and water. This is the reaction taking place in the well-known "volcano" that occurs when the household products, baking soda and vinegar, are combined.
"You could be getting tons of sodium in your system through the sodium bicarbonate so.. IDK." - so far the ACV/BS combo is minimizing my symptoms...
Last edited:
B
Braveheart
Guest
huh?I stand corrected Travis you are correct:
Vinegar is a liquid consisting of about 5–20% acetic acid (CH3COOH), water (H2O)
An acetate /ˈæsɪteɪt/ is a salt formed by the combination of acetic acid with an alkaline, earthy, metallic or nonmetallic and other base.
The acetate anion, [CH3COO]−,(or [C2H3O2]−) is one of the carboxylate family. It is the conjugate base of acetic acid. Above a pH of 5.5, acetic acid converts to acetate:[2]
CH3COOH ⇌ CH3COO− + H+
Many acetate salts are ionic, indicated by their tendency to dissolve well in water. A commonly encountered acetate in the home is sodium acetate, a white solid that can be prepared by combining vinegar and sodium bicarbonate ("bicarbonate of soda"):
Sodium acetate, CH3COONa, also abbreviated NaOAc,[8] is the sodium salt of acetic acid
Sodium bicarbonate (IUPAC name: sodium hydrogen carbonate), commonly known as baking soda, is a chemical compound with the formula NaHCO3. It is a salt composed of sodium ions and bicarbonate ions.
Sodium bicarbonate mixed with water can be used as an antacid to treat acid indigestion and heartburn.[27] Its reaction with stomach acid produces salt, water, and carbon dioxide:
Sodium bicarbonate reacts with acetic acid (found in vinegar), producing sodium acetate, water, and carbon dioxide:
Acetate is a common anion in biology. It is mainly utilized by organisms in the form of acetyl coenzyme A.
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism.[1] Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production. Coenzyme A (CoASH or CoA) consists of a β-mercaptoethylamine group linked to the vitamin pantothenic acid through an amide linkage [2] and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol).
CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates through glycolysis and by the breakdown of fatty acids through β-oxidation. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is oxidized to carbon dioxide and water, and the energy released captured in the form of 11 ATP and one GTP per acetyl group.
At high glucose levels, acetyl-CoA is produced through glycolysis.[10] Pyruvate undergoes oxidative decarboxylation in which it loses its carboxyl group (as carbon dioxide) to form acetyl-CoA, giving off 33.5 kJ/mol of energy. The oxidative conversion of pyruvate into acetyl-CoA is referred to as the pyruvate dehydrogenase reaction. It is catalyzed by the pyruvate dehydrogenase complex.
At low glucose levels, the production of acetyl-CoA is linked to β-oxidation of fatty acids. Fatty acids are first converted to acyl-CoA. Acyl-CoA is then degraded in a four-step cycle of dehydrogenation, hydration, oxidation and thiolysis catalyzed by four respective enzymes, namely acyl-CoA dehydrogenase, enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and thiolase. The cycle produces a new acyl-CoA with two fewer carbons and acetyl-CoA as a byproduct.[11]
Acetyl-CoA reacts with oxaloacetate to form citrate, which is then oxidized to CO2 in the cycle
Acetyl-CoA is also the source of the acetyl group incorporated onto certain lysine residues of histone and nonhistone proteins in the posttranslational modification acetylation. This acetylation is catalyzed by acetyltransferases. This acetylation affects cell growth, mitosis, and apoptosis.[18]
- Acetyl-CoA serves as an allosteric regulator of pyruvate dehydrogenase kinase (PDK). It regulates through the ratio of acetyl-CoA versus CoA. Increased concentration of acetyl-CoA activates PDK.[19]
- Acetyl-CoA is also an allosteric activator of pyruvate carboxylase.[
B
Braveheart
Guest
Thanks...this I can comprehend...Per Ray:
Warburg, Koch, and Szent-Gyorgyi had a comprehensive view of biology, in which the aerobic production of lactate, resulting from a respiratory defect, itself was functionally related to the nature of cancer. Carbon Dioxide opposes Lactate.
Sodium bicarbonate reacts with acetic acid (found in vinegar), producing sodium acetate, water, and carbon dioxide:
At high glucose levels, acetyl-CoA is produced through glycolysis.[10] Pyruvate undergoes oxidative decarboxylation in which it loses its carboxyl group (as carbon dioxide)
But what is interesting is At low glucose levels, the production of acetyl-CoA is linked to β-oxidation of fatty acids. No carbon dioxide. Case for NOT limiting glucose which Ray has mentioned many times
Acetyl-CoA reacts with oxaloacetate to form citrate, which is then oxidized to CO2 in the cycle. More Carbon dioxide!
But since cancer is a respiratory defect I think it gobbles up glucose and no Acetyl-CoA is produced and Pyruvate doe not undergoes oxidative decarboxylation so it builds up and converts to Lactate. Acetate would fix this...via CO2...
Now add potassium ascorbate to the picture. Per Ted -As for the ascorbate, which is an alkaline form of vitamin C, it kills them again by their negative millivolts. Ascorbate kills them with the -200 or -100 millivolts depending on the dose. and the potassium itself is toxic to cancer cells
OP
TreasureVibe
Member
- Joined
- Jul 3, 2016
- Messages
- 1,941
Ofcourse I estimate your knowledge on health higher now, and I didn't mean with that quote to advice those things too. I just put it in because just like Ted, Budwig can say some good things, for example sodium displacing potassium in cells.I took Flax seed oil and fish oil every day before my PSA hit 13,000. NOT!!!!
"Tried smelling the substance while it was fizzing, and a toxic smelling, high-making gas came off it, which made me feel lightheaded upon inhalation"
- For laboratory use, sodium acetate is inexpensive and usually purchased instead of being synthesized. It is sometimes produced in a laboratory experiment by the reaction of acetic acid, commonly in the 5–8% solution known as vinegar, with sodium carbonate ("washing soda"), sodium bicarbonate ("baking soda"), or sodium hydroxide ("lye", or "caustic soda"). Any of these reactions produce sodium acetate and water. When a sodium and carbonate ion-containing compound is used as the reactant, the carbonate anion from sodium bicarbonate or carbonate, reacts with hydrogen from the carboxyl group (-COOH) in acetic acid, forming carbonic acid. Carbonic acid readily decomposes under normal conditions into gaseous carbon dioxide and water. This is the reaction taking place in the well-known "volcano" that occurs when the household products, baking soda and vinegar, are combined.
"You could be getting tons of sodium in your system through the sodium bicarbonate so.. IDK." - so far the ACV/BS combo is minimizing my symptoms...
Btw - did you fact-check the benzoic acid in Oxidal? (WHO saying when mixed with vitamin C/carbonated drink it makes benzene which is a carcinogen)
Cancer can be a silent disease. Alot of people feel fine and don't notice anything, just before getting a diagnose of cancer. Don't go by symptoms alone. Keep your head with the program. Sodium bicarbonate might be fluxing the system with alot of extra unused sodium ions. These sodium ions can displace potassium in healthy cells making them vulnerable for disease, like cancer. Dr. Pantellini reported improved quality of life in his cancer patients using potassium ascorbate alone. So it might just be the potassium acetate that's making you feel good. You should think of trying potassium bicarbonate and potassium acetate to see if that gives the same effects.
BTW - Doesn't potassium and sodium acetate simply breaks into a sodium ion or a potassium ion and acetate upon entering the bloodstream, rendering this whole treatment useless?
Last edited:
OP
TreasureVibe
Member
- Joined
- Jul 3, 2016
- Messages
- 1,941
The human organism is, in embryonic life and early infancy, a sodium-animal, due to the relative preponderance of Na throughout the entire organism, but in adult life, a potassium-animal. The potassium predominance must be maintained throughout life.
Source: https://raypeatforum.com/community/threads/a-cancer-therapy-by-max-gerson-selected-parts.13998/
@Amazoniac
The official RDA for potassium is 4700 mg...
Source: https://raypeatforum.com/community/threads/a-cancer-therapy-by-max-gerson-selected-parts.13998/
@Amazoniac
The official RDA for potassium is 4700 mg...
Last edited:
EMF Mitigation - Flush Niacin - Big 5 Minerals
Similar threads
- Replies
- 33
- Views
- 13K
- Replies
- 2
- Views
- 3K
- Replies
- 13
- Views
- 5K
- Replies
- 73
- Views
- 21K
- Replies
- 22
- Views
- 12K
- Replies
- 11
- Views
- 4K
- Replies
- 0
- Views
- 1K
