Tristan Loscha
Member
- Joined
- Dec 18, 2018
- Messages
- 2,206
Firstly i never directly drink water only whats found in OJ, milk, coffee or foods. Secondly I dont urinate that often actually and its never clear like the 'healthy' people. Thirdly, as before, in the context of the average diet, any nutrient can be proven to he 'harmfull' but its not really proof at all. In a nutrient dense diet, one doesnt need to use NaCl sparingly. Thats context and its the RP way.
Im not recommending the average sheeple increase its intake or sugar or any other nutrient because basically they are FUBAR.
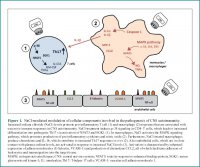
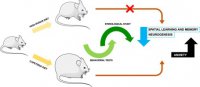
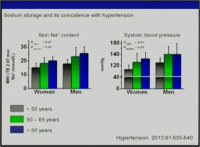
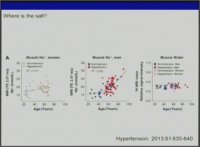
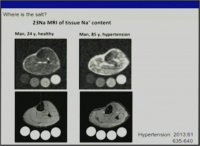
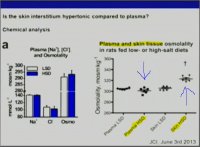
..Yet it seems that for Sodium,it isnt a relative effect,like the observed balance-like relation between Sodium and Potassium.
It is an absolute effect,and one cant discern if one is salt sensitive or salt resistive,
or has metabolic milieu that encourages salt-loading.In a "nutrient dense diet",if these findings hold true,based on metabolic make-up,you do have to moderate NaCl intake,because you consume otherwise supraphysiologic amount of this mineral,
and it has messenger effect and toxicity effect.Ask yourself the question,why you would hesitate to consume 10-to-20-fold
of zinc,iron or Potassium-salts.After all,you can,buy it from ebay,and then just load up your dispenser Bro!