md_a
Member
- Joined
- Aug 31, 2015
- Messages
- 468
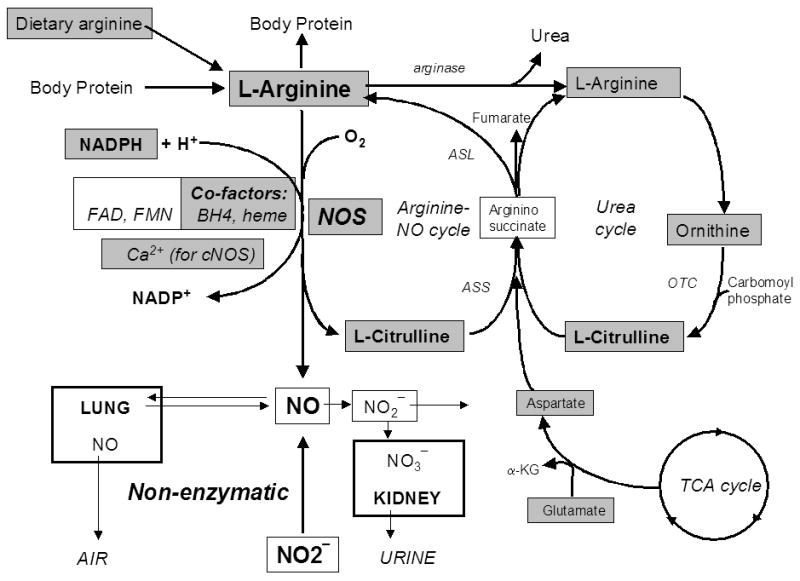
Aside from the anti-inflammatory stuff, I think two important methods as prevention / treatment against covid is to block the virus from attaching to ACE2 and to reduce the the angiotensin II type 1 receptor (AT1 receptor) in preventing the virus from entering the cell.
..........
The mechanistic overview of SARS-CoV-2 using angiotensin-converting enzyme 2 to enter the cell for replication: possible treatment options related to the renin–angiotensin system
..........
Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction - PubMed
.........
Methods: Chronic CsA nephropathy was induced by administering CsA (30 mg/kg) to mice on a low-salt diet (LSD) for 4 weeks. A normal-salt diet (NSD) was used as the control. Reverse transcription-polymerase chain reaction, western blot and immunohistochemistry were performed for Klotho and intrarenal RAS activity was measured using immunohistochemistry for angiotensinogen and renin. Oxidative stress was measured with urinary excretion of 8-hydroxy-2'-deoxyguanosine (8-OHdG).
Results: CsA treatment decreased Klotho mRNA and protein in mouse kidney in a dose-dependent and time-dependent manner, but a concurrent treatment with losartan, an angiotensin II type 1 (AT1) receptor blocker, reversed the decrease in Klotho expression with histological improvement. This finding was more marked in the LSD than the NSD. Klotho expression was correlated with angiotensinogen and renin expression, tubulointerstitial fibrosis score and urinary 8-OHdG excretion.
Conclusions: Angiotensin II may play a pivotal role in regulating Klotho expression in CsA-induced renal injury. AT1 receptor blocker may inhibit the ageing process by decreasing oxidative stress caused by CsA.

 pubmed.ncbi.nlm.nih.gov
..............
pubmed.ncbi.nlm.nih.gov
..............
View: https://www.youtube.com/watch?v=JvqgiMuRRRY
..........
The mechanistic overview of SARS-CoV-2 using angiotensin-converting enzyme 2 to enter the cell for replication: possible treatment options related to the renin–angiotensin system
Abstract
The SARS-CoV-2 pandemic is a healthcare crisis caused by insufficient knowledge applicable to effectively combat the virus. Therefore, different scientific discovery strategies need to be connected, to generate a rational treatment which can be made available as rapidly as possible. This relies on a solid theoretical understanding of the mechanisms of SARS-CoV-2 infection and host responses, which is coupled to the practical experience of clinicians that are treating patients. Because SARS-CoV-2 enters the cell by binding to angiotensin-converting enzyme 2 (ACE2), targeting ACE2 to prevent such binding seems an obvious strategy to combat infection. However, ACE2 performs its functions outside the cell and was found to enter the cell only by angiotensin II type 1 receptor (AT1R)-induced endocytosis, after which ACE2 is destroyed. This means that preventing uptake of ACE2 into the cell by blocking AT1R would be a more logical approach to limit entry of SARS-CoV-2 into the cell. Since ACE2 plays an important protective role in maintaining key biological processes, treatments should not disrupt the functional capacity of ACE2, to counterbalance the negative effects of the infection. Based on known mechanisms and knowledge of the characteristics of SARS-CoV we propose the hypothesis that the immune system facilitates SARS-CoV-2 replication which disrupts immune regulatory mechanisms. The proposed mechanism by which SARS-CoV-2 causes disease immediately suggests a possible treatment, since the AT1R is a key player in this whole process. AT1R antagonists appear to be the ideal candidate for the treatment of SARS-CoV-2 infection. AT1R antagonists counterbalance the negative consequences of angiotesnin II and, in addition, they might even be involved in preventing the cellular uptake of the virus without interfering with ACE2 function. AT1R antagonists are widely available, cheap, and safe. Therefore, we propose to consider using AT1R antagonists in the treatment of SARS-CoV-2.Conclusion
Because SARS-CoV-2 enters the cell bound to ACE2, which induces ACE2 deficiency at the cell membrane, AngII is persistently activated. Increased AngII induces activation of AT1R, causing more uptake of SARS-CoV-2 and increasing ACE2 deficiency, thus maintaining and exacerbating a non-specific immune response, consisting of cytokine-induced inflammation. This non-specific immune response is an attempt to reduce the viral load, while the specific immune response is mounted. Unrestrained AngII eventually causes death by respiratory distress induced by excessive inflammation and its deleterious effects on other organs. Therefore, SARS-CoV-2-induced mortality is promoted by three mechanisms: (i) increased AngII induces endocytosis of ACE2-bound SARS-CoV-2, leading to ACE2 deficiency and viral replication; (ii) ACE2 deficiency prevents the priming of an adaptive immune response by lack of NO; and (iii) Ang II induces an increase in viral load leading to an increased innate immune response and a further increase in AngII levels. Therefore, treatments should aim at preventing the AngII ‘storm’ in an early phase of the infection, restoring the modulation of NO, and preventing the entry of SARS-CoV-2 into the cell. All these mechanisms are targeted by AT1R antagonists. They may reduce morbid inflammatory distress and provide an environment to facilitate an effective, virus-specific adaptive immune response.The mechanistic overview of SARS-CoV-2 using angiotensin-converting enzyme 2 to enter the cell for replication: possible treatment options related to the renin–angiotensin system
..........
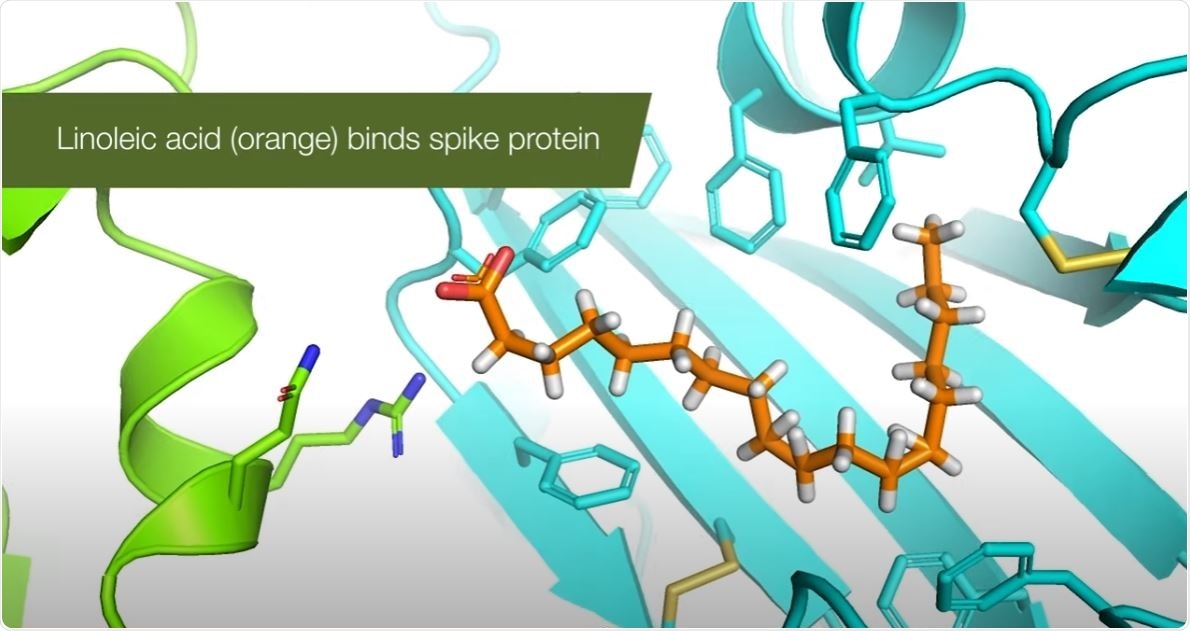
Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction
Abstract
Severe acute respiratory syndrome (SARS) is an emerging infectious disease caused by a novel coronavirus (SARS-CoV). SARS-CoV spike (S) protein, a type I membrane-bound protein, is essential for the viral attachment to the host cell receptor angiotensin-converting enzyme 2 (ACE2). By screening 312 controlled Chinese medicinal herbs supervised by Committee on Chinese Medicine and Pharmacy at Taiwan, we identified that three widely used Chinese medicinal herbs of the family Polygonaceae inhibited the interaction of SARS-CoV S protein and ACE2. The IC(50) values for Radix et Rhizoma Rhei (the root tubers of Rheum officinale Baill.), Radix Polygoni multiflori (the root tubers of Polygonum multiflorum Thunb.), and Caulis Polygoni multiflori (the vines of P. multiflorum Thunb.) ranged from 1 to 10 microg/ml. Emodin, an anthraquinone compound derived from genus Rheum and Polygonum, significantly blocked the S protein and ACE2 interaction in a dose-dependent manner. It also inhibited the infectivity of S protein-pseudotyped retrovirus to Vero E6 cells. These findings suggested that emodin may be considered as a potential lead therapeutic agent in the treatment of SARS.Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction - PubMed
.........
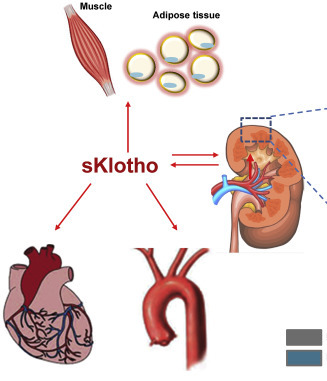
Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy
Abstract
Background: The Klotho gene plays a role in suppressing ageing-related disorders. It is suggested that activation of renin-angiotensin system (RAS) or oxidative stress suppresses Klotho in the kidney. This study evaluated the association between Klotho expression and RAS in cyclosporine (CsA)-induced renal injury.Methods: Chronic CsA nephropathy was induced by administering CsA (30 mg/kg) to mice on a low-salt diet (LSD) for 4 weeks. A normal-salt diet (NSD) was used as the control. Reverse transcription-polymerase chain reaction, western blot and immunohistochemistry were performed for Klotho and intrarenal RAS activity was measured using immunohistochemistry for angiotensinogen and renin. Oxidative stress was measured with urinary excretion of 8-hydroxy-2'-deoxyguanosine (8-OHdG).
Results: CsA treatment decreased Klotho mRNA and protein in mouse kidney in a dose-dependent and time-dependent manner, but a concurrent treatment with losartan, an angiotensin II type 1 (AT1) receptor blocker, reversed the decrease in Klotho expression with histological improvement. This finding was more marked in the LSD than the NSD. Klotho expression was correlated with angiotensinogen and renin expression, tubulointerstitial fibrosis score and urinary 8-OHdG excretion.
Conclusions: Angiotensin II may play a pivotal role in regulating Klotho expression in CsA-induced renal injury. AT1 receptor blocker may inhibit the ageing process by decreasing oxidative stress caused by CsA.

Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy - PubMed
Angiotensin II may play a pivotal role in regulating Klotho expression in CsA-induced renal injury. AT1 receptor blocker may inhibit the ageing process by decreasing oxidative stress caused by CsA.
Ray Peat klotho protein (anti-aging) similar to Vitamin D.
View: https://www.youtube.com/watch?v=JvqgiMuRRRY