“ferric ammonium citrate-induced interleukin-1b production is dependent on caspase-1 and is significantly inhibited by an Fe2+-specific chelator. Ferric ammonium citrate consistently induced interleukin-1b secretion in THP1 cells, but not in NLRP3-deficient THP1 cells, indicating a requirement for the NLRP3 inflammasome. Additionally, activation of the inflammasome is mediated by potas- sium efflux, reactive oxygen species-mediated mitochondrial dysfunction, and lysosomal membrane permeabilization. Thus, these results suggest that monocytes/macrophages not only sequestrate iron during inflammation, but also mediate inflammation in response to cellular labile iron, which provides novel insights into the role of iron in chronic inflammation.”
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
-
By using this site you agree to the terms, rules, and privacy policy.
-
Charlie's Restoration Giveaway #2 (Entire Home EMF Mitigation & Protection Along With Personal Protection) - Click Here To Enter
-
Dear Carnivore Dieters, A Muscle Meat Only Diet is Extremely Healing Because it is a Low "vitamin A" Diet. This is Why it Works so Well...
Rest the rest of this post by clicking here
-
The Forum is transitioning to a subscription-based membership model - Click Here To Read
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Activation of the NLRP3 inflammasome by cellular labile iron
- Thread starter Jamsey
- Start date

Effect of angiotensin II on iron-transporting protein expression and subsequent intracellular labile iron concentration in human glomerular endothelial cells - PubMed
Angiotensin II (Ang II)-induced endothelial injury, which is associated with atherosclerosis, is believed to be mediated by intracellular reactive oxygen species (ROS) through stimulation of nicotinamide adenine dinucleotide phosphate oxidase (NOX). Iron is essential for the amplification of...

JNK/p66Shc/ITCH Signaling Pathway Mediates Angiotensin II-induced Ferritin Degradation and Labile Iron Pool Increase
Angiotensin II (Ang II) induces deleterious changes in cellular iron metabolism and increases the generation of reactive oxygen species. This leads to an impairment of neuronal and vascular function. However, the mechanism underpinning Ang II-induced ...
“Ang II-induced ferritin degradation and, hence, elevation of the levels of highly reactive iron, are mediated by the JNK/p66Shc/ITCH signaling pathway.”

The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation
The NLRP3 inflammasome is a critical component of the innate immune system that mediates caspase-1 activation and the secretion of proinflammatory cytokines IL-1β/IL-18 in response to microbial infection and cellular damage. However, the aberrant ...
“IL-18 is necessary for interferon-gamma (IFN-γ) production”
Interleukin-18 and Interleukin-1β: Two Cytokine Substrates for ICE (Caspase-1) - Journal of Clinical Immunology
This special article deals with the role of processing enzymes in the generation of bioactive cytokines, particularly IL-1β and the novel cytokine IL-18, which was formerly called IFNγ-inducing factor (IGIF). The “classical” pathways of cytokine processing are described, as well as the...

Interleukin-18 and IL-18 Binding Protein
Interleukin-18 (IL-18) is a member of the IL-1 family of cytokines. Similar to IL-1β, IL-18 is synthesized as an inactive precursor requiring processing by caspase-1 into an active cytokine but unlike IL-1β, the IL-18 precursor is constitutively present in nearly all cells in healthy humans and...
And if you think this is too far down the chain,
“Aldo, endothelin-1 (ET-1), and angiotensin II (ANG II) increased IL-18 mRNA and protein expression.”
“Our findings indicate that Aldo induces IL-18 expression through a mechanism that involves, at a minimum, ET-1 and ANG II acting via the Rho/Rho-kinase and PPAR/NF-κB pathway.”
“Atherosclerotic lesion development was reduced in apo E−/− mice either by overexpressing IL-18 binding protein, the endogenous inhibitor of IL-188, or by IL-18 deficiency”
So to summarize so far,
Angiotensin II -> Increased cellular iron -> nlrp3 inflammasome activation-> Caspase-1 activation -> il-18 activation -> interferon gamma induction
Last edited:
I find the caspase-1 dependent induction of il-18(and subsequently interferon gamma) and il-1b interesting as it fits directly into the theories of Travis relating to hair loss.
 www.mdpi.com
“The very same research study identified the proinflammatory cytokines, IFNγ and TNFα, as regulators of PRL expression in HFs. Interestingly, dopamine, known as an inhibitor of PRL pituitary secretions, has no effect on PRL or PRL-R expression in human HFs [22]”
www.mdpi.com
“The very same research study identified the proinflammatory cytokines, IFNγ and TNFα, as regulators of PRL expression in HFs. Interestingly, dopamine, known as an inhibitor of PRL pituitary secretions, has no effect on PRL or PRL-R expression in human HFs [22]”

 www.ncbi.nlm.nih.gov
“Interferon (IFN) γ increased PRL IR in the epithelium of human HFs”
www.ncbi.nlm.nih.gov
“Interferon (IFN) γ increased PRL IR in the epithelium of human HFs”
As a side note, local hair follicle prolactin receptors have been implicated as a cause of hair loss on this forum and interferon gamma appears to be the main cause.I think it could be the thymus gland, mostly, but also perhaps the adrenals.
Hamilton made many observations in the '50s about androgens and hair loss. His arguments weren't biochemical; they were mostly epidemiological, and observational. When males are castrated young, they are largely protected from hair loss; however, castrating a male after the year 18 has little effect.
I read a few old studies on what happens to rats under these conditions. Early castration changes the entire adrenal output. The adrenals have androgen receptors, and they increase in size as a result of stimulation early in development. Androgens are always anabolic, and can never cause hair loss directly. Not even Phizer can't argue this point, as Finansteride® needs to be taken internally to have any effect at all. This way, they tacitly admit what I'm saying. Spironolactone works topically, and is more of a antimineralcorticoid than an antiandrogen.
Interferon-γ is produced nearly exclusively from T-cells, or cells derived from the thymus. Here, again, androgen acts anabolically to differentiate the thymus from the female phenotype. Males have a different immune parameters, something I had just found out yesterday. I haven't read, for example, studies such as the one listed below yet.
Giron-Gonzalez, J. A., et al. "Consistent production of a higher TH1: TH2 cytokine ratio by stimulated T cells in men compared with women." European journal of endocrinology (2000)
I am willing to predict that you'd find higher amounts of interferon-γ in males. I consider this the public enemy №1 of cytokines, with interleukin-1β public enemy №2. Both these cytokines induce transcription of phospholipase A₂, and rats genetically-engineered to over-express this on the skin are bald just as you'd expect. Rats genetically-engineered to over-express interferon-γ are similarly bald, as are the ones over-expressing cyclooxygenase-2. Don't believe me? then do some GoogleScholararing: Google Scholar
Louis Garza, when analyzing lipids (not cytokines or steroids) found that prostaglandin D₂ was synonymous with hair loss. I have just found that prostaglandin D₂ is a high-affinity ligand for the PPARα receptor, and also that this receptor has a promoter for interleukin-1β receptor (Table 2).
It might certainly seem that prostaglandins are highly involved. Perhaps hair loss can be perfectly understood, the confusion only coming from concentrating on only one correlation at once—not seeing the causality and interrelations. The most striking result from the interaction of interferon-γ and the cell is the transcription of phospholipase A₂; this happens to such an extent that you could consider this its primary function. This result from this event is, of course, a long release of prostaglandins from the cell membrane—longer than the transient stimulation from particles such as TGF-β₁.
I think someone needs to reanalyze the epidemiological correlations; perhaps plot alopecia against the linoleic acid consumption of populations. I wouldn't expect any hair loss to be observed among the coconut-eating islanders of the South Pacific, such as the ones studied by Ian Prior in his research on cardiovascular disease. I know he did a study on a migrants to New Zealand; and since he was studying cardiovascular disease, he would have kept track of the quantity and quality of lipids ingested and would have measured their blood concentrations.
Prior, Ian. "The Tokelau island migrant study." International Journal of Epidemiology (1974)

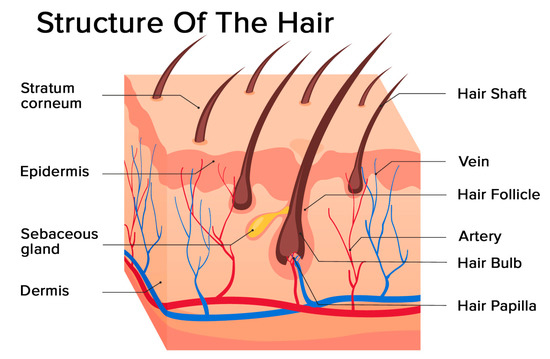
Hormonal Effects on Hair Follicles
The hair cycle and hair follicle structure are highly affected by various hormones. Androgens—such as testosterone (T); dihydrotestosterone (DHT); and their prohormones, dehydroepiandrosterone sulfate (DHEAS) and androstendione (A)—are the key factors in terminal hair growth. They act on...

Tumour Necrosis Factor Alpha, Interferon Gamma and Substance P Are Novel Modulators of Extrapituitary Prolactin Expression in Human Skin
Human scalp skin and hair follicles (HFs) are extra-pituitary sources of prolactin (PRL). However, the intracutaneous regulation of PRL remains poorly understood. Therefore we investigated whether well-recognized regulators of pituitary PRL expression, ...
So the question is how does this axis get worse with age.
Ray peat stated that “During aging, our tissues tend to store an excess of iron. There is a remarkably close association between the amount of iron stored in our tissues and the risk of death from cancer, heart disease, or from all causes. This relationship between iron and death rate exists even during childhood, but the curve is downward until the age of 12, and then it rises steadily until death. The shape of this curve, representing the iron burden, is amazingly similar to the curves representing the rate of death in general, and the rate of death from cancer. There is no other relationship in biology that I know of that has this peculiar shape, with its minimum at the age of 12, and its maximum in old age at the time of death.”
I started looking into how this iron deposition affects the angiotensin system. I found that iron overload increases the effects of angiotensin II through the at1 receptor.

 pubmed.ncbi.nlm.nih.gov
pubmed.ncbi.nlm.nih.gov
 www.sciencedirect.com
www.sciencedirect.com
Angiotensin II also induces iron deposition through the at1 receptor.

 www.ncbi.nlm.nih.gov
“Real-time polymerase chain reaction (PCR) showed that the mRNA expression of TfR, iron-responsive element–negative DMT1, FPN, and hepcidin mRNA increased ~1.9-fold, ~1.7-fold, ~2.3-fold, and ~4.7-fold, respectively, after angiotensin II infusion as compared with that of untreated controls, and that these increases could be suppressed by the concomitant administration of losartan.”
www.ncbi.nlm.nih.gov
“Real-time polymerase chain reaction (PCR) showed that the mRNA expression of TfR, iron-responsive element–negative DMT1, FPN, and hepcidin mRNA increased ~1.9-fold, ~1.7-fold, ~2.3-fold, and ~4.7-fold, respectively, after angiotensin II infusion as compared with that of untreated controls, and that these increases could be suppressed by the concomitant administration of losartan.”
So, through a vicious cycle, stress or angiotensin II increases iron retention and iron retention increases the damage of stress or angiotensin II.
Also, just to show direct evidence relating iron chelators and hair loss:
HIF-1α Stimulators Function Equally to Leading Hair Loss Agents in Enhancing Dermal Papilla Growth
Other studies I found interesting.

 www.ncbi.nlm.nih.gov
“We report that excess iron, but not other Fenton catalytic metals, induces activation of the NLRP3 inflammasome, a pathway also implicated in AMD.”
www.ncbi.nlm.nih.gov
“We report that excess iron, but not other Fenton catalytic metals, induces activation of the NLRP3 inflammasome, a pathway also implicated in AMD.”
 “Many life-extending interventions, such as rapamycin, calorie restriction, and old plasma dilution can be explained by the effects they have on iron absorption, excretion, and metabolism.”
“Many life-extending interventions, such as rapamycin, calorie restriction, and old plasma dilution can be explained by the effects they have on iron absorption, excretion, and metabolism.”
“Moreover, unlike transferrin iron uptake, which is inhibited at high tissue iron concentrations by down-regulation of transferrin receptor production, nontransferrin iron uptake is increased by high tissue iron content [23].”
Ray peat stated that “During aging, our tissues tend to store an excess of iron. There is a remarkably close association between the amount of iron stored in our tissues and the risk of death from cancer, heart disease, or from all causes. This relationship between iron and death rate exists even during childhood, but the curve is downward until the age of 12, and then it rises steadily until death. The shape of this curve, representing the iron burden, is amazingly similar to the curves representing the rate of death in general, and the rate of death from cancer. There is no other relationship in biology that I know of that has this peculiar shape, with its minimum at the age of 12, and its maximum in old age at the time of death.”
I started looking into how this iron deposition affects the angiotensin system. I found that iron overload increases the effects of angiotensin II through the at1 receptor.

Blockade of angiotensin AT1 receptors prevents arterial remodelling and stiffening in iron-overloaded rats - PubMed
Losartan prevented the structural and functional indices of aortic stiffness in iron-overloaded rats, implying that inhibition of the renin-angiotensin system would limit the vascular remodelling in chronic iron-overload.

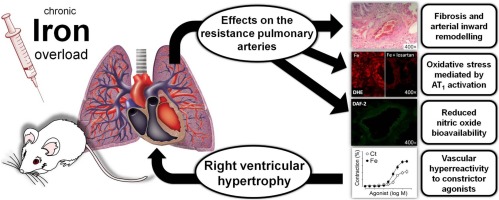
Chronic iron overload induces vascular dysfunction in resistance pulmonary arteries associated with right ventricular remodeling in rats
Although iron excess is toxic to the vasculature and even that pulmonary hypertension has been reported in this scenario, the role of iron overload pe…
Angiotensin II also induces iron deposition through the at1 receptor.

Angiotensin II promotes iron accumulation and depresses PGI2 and NO synthesis in endothelial cells: effects of losartan and propranolol analogs
Angiotensin may promote endothelial dysfunction through iron accumulation. To research this, bovine endothelial cells (ECs) were incubated with iron (30 μmol·L[−1] ) with or without angiotensin II (100 nmol·L[−1] ...
So, through a vicious cycle, stress or angiotensin II increases iron retention and iron retention increases the damage of stress or angiotensin II.
Also, just to show direct evidence relating iron chelators and hair loss:
HIF-1α Stimulators Function Equally to Leading Hair Loss Agents in Enhancing Dermal Papilla Growth
Other studies I found interesting.

Iron toxicity in the retina requires Alu RNA and the NLRP3 inflammasome
Excess iron induces tissue damage and is implicated in age-related macular degeneration (AMD). Iron toxicity is widely attributed to hydroxyl radical formation through Fenton's reaction. We report that excess iron, but not other Fenton catalytic metals, ...

Iron: an underrated factor in aging | Aging
Aging | doi:10.18632/aging.203612. Dennis Mangan
www.aging-us.com
“Moreover, unlike transferrin iron uptake, which is inhibited at high tissue iron concentrations by down-regulation of transferrin receptor production, nontransferrin iron uptake is increased by high tissue iron content [23].”
EMF Mitigation - Flush Niacin - Big 5 Minerals
Similar threads
- Replies
- 1
- Views
- 759
- Replies
- 2
- Views
- 2K
P
- Replies
- 15
- Views
- 5K
- Replies
- 128
- Views
- 25K
- Replies
- 4
- Views
- 6K
- Replies
- 0
- Views
- 5K
- Replies
- 602
- Views
- 211K
- Replies
- 14
- Views
- 3K
