hang loose
Member
- Joined
- Mar 5, 2016
- Messages
- 10
Hi Travis,
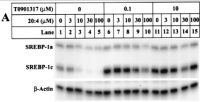
I posted the following already in another thread. Maybe it’s better to ask it here. I am just super curious about more info on acne.
Many other users and readers are interested as well, IMO.
„Thanks for sharing your thoughts and research on acne.
I also found those studies linking acne to an imbalance of Lipids some time ago.
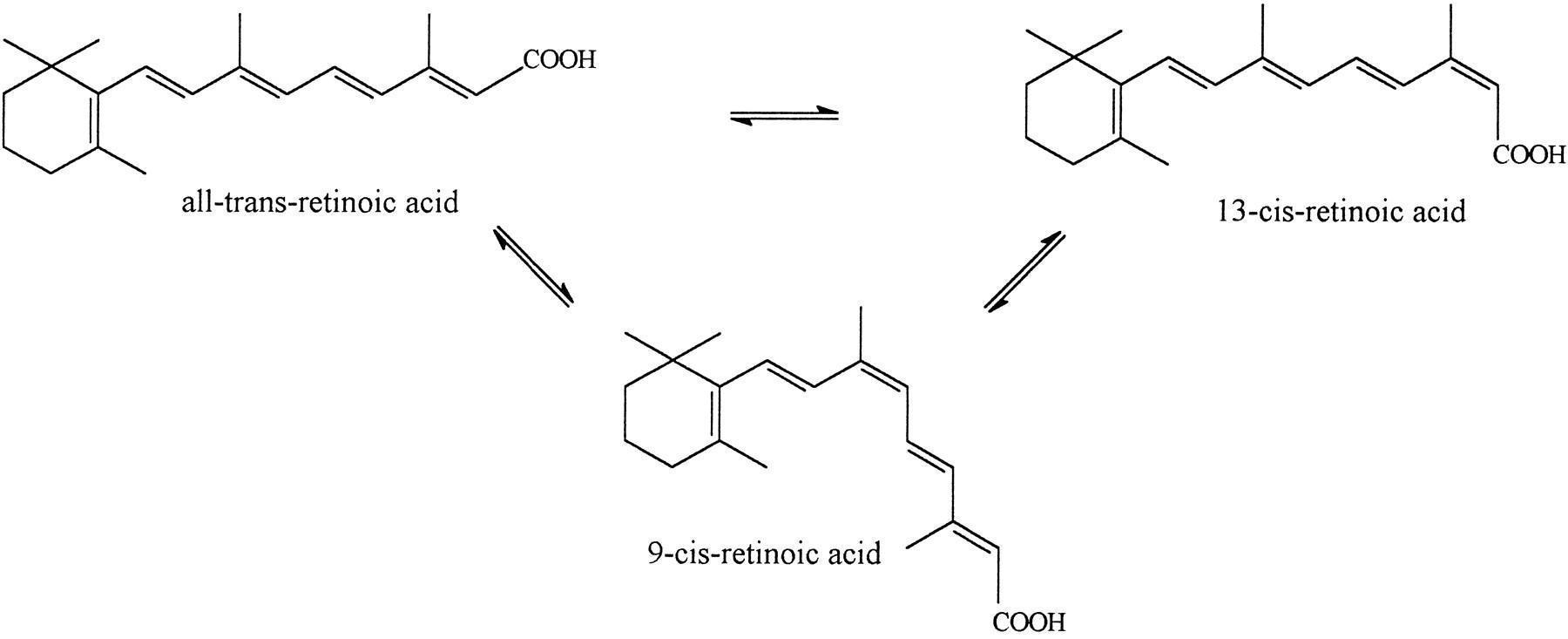
Dr. Peat has said a couple of times that supplementing thyroid and vitamin A (or eating liver on a weekly basis) will (most likely) clear acne.
What are your thoughts on this?
I would be very grateful about your opinion.“
I posted the following already in another thread. Maybe it’s better to ask it here. I am just super curious about more info on acne.
Many other users and readers are interested as well, IMO.
„Thanks for sharing your thoughts and research on acne.
I also found those studies linking acne to an imbalance of Lipids some time ago.
Dr. Peat has said a couple of times that supplementing thyroid and vitamin A (or eating liver on a weekly basis) will (most likely) clear acne.
What are your thoughts on this?
I would be very grateful about your opinion.“