Limon9
Member
The role of trimethylamine n-oxide (TMAO) in human disease has been quite controversial. The carnitine/choline in red meat and eggs had been purported to generate trimethylamine (TMA) via bacterial metabolism, which then goes on to become TMAO via the action of hepatic FMO3. The often-promoted "heart healthy" fishes contain intact TMAO which is also readily absorbed. Studies and trials consistently find that an increased amount of Firmicutes and decreased Bacteroidetes to be a marker for elevated TMAO production. This experiment found that simply giving vitamin D3 was enough to greatly reduce the rise in TMAO. The authors also note that testosterone seems to suppress FMO3 activity, consistent with estrogen inducing phosphatidylethanolamine methyltransferase, the "choline-synthesizing" enzyme, thereby reducing the dietary CHO requirement of women. Eggs are widely-considered a "testosterone food", having cholesterol, "vitamin" A and other nutrients, and a deficiency of this hormone is known to incline one to many diseases. Vitamin D3 seems to orient all of these things in a coherent direction, and might economize the choline requirement by preventing its bacterial wastage. So... it should help the liver.
Vitamin D: A New Player In Non-alcoholic Fatty Liver Disease?
Supplementary Choline Raises Risk Of Blood Clots; Aspirin To The Rescue
It is noted that the control diet was AIN-93G, containing 1g/kg of choline (0.1%), and the high-choline groups were given AIN-93G plus one percent choline, or 1.1%. (Seems like a lot)
Key Points
- 5 groups: control (C), high-choline (HC), control with D3 (CD3), high-choline with D3 (HCD3), and high-choline with D3 and antibiotics (HCD3A)
- C and CD3 were not very different.
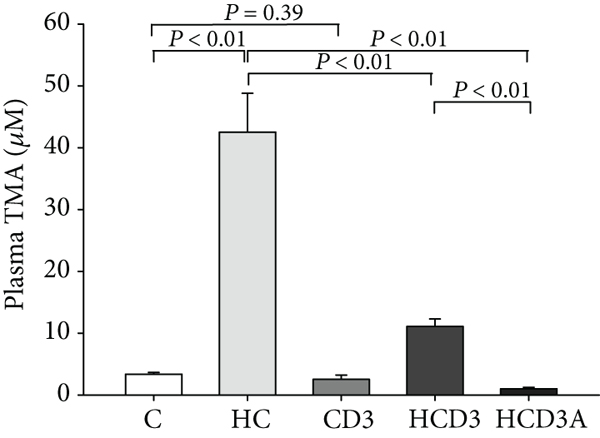
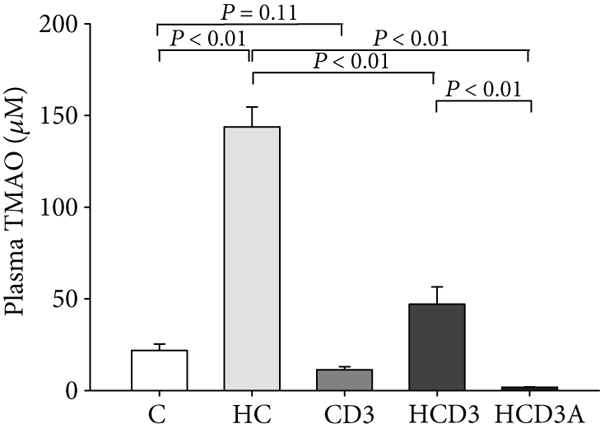
- HCD3 significantly ameliorated the elevated TMA/TMAO levels seen in the HC group.
- HCD3A almost completely abolished TMAO levels.
- Levels of FMO3 were not significantly different between groups.
- The amount of low-TMA-producing Bacteroidetes were diminished in a high-choline diet, with elevated levels of Firmicutes.
Vitamin D Decreases Plasma Trimethylamine-N-oxide Level in Mice by Regulating Gut Microbiota
Biomed Res Int . 2020 Oct 5;2020:9896743. Wang X, Li X, Dong Y.
Highlights
Vitamin D: A New Player In Non-alcoholic Fatty Liver Disease?
Supplementary Choline Raises Risk Of Blood Clots; Aspirin To The Rescue
It is noted that the control diet was AIN-93G, containing 1g/kg of choline (0.1%), and the high-choline groups were given AIN-93G plus one percent choline, or 1.1%. (Seems like a lot)
Key Points
- 5 groups: control (C), high-choline (HC), control with D3 (CD3), high-choline with D3 (HCD3), and high-choline with D3 and antibiotics (HCD3A)
- C and CD3 were not very different.
- HCD3 significantly ameliorated the elevated TMA/TMAO levels seen in the HC group.
- HCD3A almost completely abolished TMAO levels.
- Levels of FMO3 were not significantly different between groups.
- The amount of low-TMA-producing Bacteroidetes were diminished in a high-choline diet, with elevated levels of Firmicutes.
Vitamin D Decreases Plasma Trimethylamine-N-oxide Level in Mice by Regulating Gut Microbiota
Biomed Res Int . 2020 Oct 5;2020:9896743. Wang X, Li X, Dong Y.
Highlights
"TMAO can enhance platelet aggregation and promote thrombosis by increasing calcium release from the endoplasmic reticulum of platelet cells [8]. Remarkably, deleting gut microbiota using broad-spectrum antibiotics eliminates the production of TMAO and decreases AS in ApoE−/− mice [9]. Therefore, CVD could be effectively prevented or treated by reducing plasma TMAO, and gut microbiota is critical to the pathogenesis of AS induced by TMAO and may become an important regulatory target."
"Clinical research has shown that a lack of vitamin D leads to an increased risk of AS and resulting CVD, such as myocardial infarction, coronary heart disease, or heart failure, which may be linked to inflammatory response, vascular calcification, impaired endothelial function, myocardial cell hypertrophy, and dysregulation of the renin-angiotensin system [11–13]. Although the mechanism is not yet fully understood, deficiency in vitamin D is regarded as a potential risk factor for CVD. Animal studies have demonstrated that oral supplementation with vitamin D can suppress chronic inflammation in the arterial wall and reduce AS by changing the differentiation or function of regulatory T cells and dendritic cells [14]."
"Fifty C57BL/6J mice (female, 6−8 weeks, procured from Beijing Vital River Laboratory Animal Technology Co., Ltd.) were housed under a strict 12 h/12 h light/dark cycle at ambient temperature (18−24°C) and comfortable humidity (50–60%). The initial body weight was 18.2 ± 1.1 g. The mice were provided with sufficient water and food. The food intake and body weight of the mice were recorded weekly over the experimental period.
. . .
After 16 weeks of treatment, mice were euthanized following a 12 h fasting period. Serum was collected by centrifugation and maintained at −80°C. The liver and the cecal contents were immediately collected and stored in liquid nitrogen at −80°C until future use."
"No statistical differences were detected in terms of food intake, body weight, or serum lipid parameters (including TC, TG, LDL-C, and HDL-C) among the five groups (P > 0.05). However, the serum 25(OH)D level was much higher in the groups supplemented with vitamin D3 [duh] (CD3, 62.03 ± 9.26 ng/mL; HCD3, 59.45 ± 4.38 ng/mL; and HCD3A, 51.07 ± 8.02 ng/mL) than both the control (C, 26.38 ± 3.41 ng/mL) and high-choline (HC, 27.71 ± 5.95 ng/mL) groups (P < 0.01)."
"After 16 weeks of treatment, dietary choline significantly increased the level of plasma TMA in group HC as compared with the C group (P < 0.01; Figure 1(a)). The plasma level of TMA in the HCD3 group was 11.09 ± 1.21 μM, which was much lower than that in the HC group (42.52 ± 6.27 μM, P < 0.01). No significant difference was found between groups C and CD3 (3.36 ± 0.29 μM vs. 2.54 ± 0.68 μM, P = 0.39). While the TMAO level was substantially elevated in mice fed a high-choline diet, vitamin D3 supplementation markedly inhibited this increase (C, 21.81 ± 3.58 μM; HC, 143.74 ± 10.86 μM; and HCD3, 47.03 ± 9.52 μM, P < 0.01; Figure 1(b)). Additionally, the antibiotic-treated group (HCD3A) had significantly lower plasma TMA and TMAO levels than the HC group (1.01 ± 0.23 μM vs. 42.52 ± 6.27 μM, 1.76 ± 0.19 μM vs. 143.74 ± 10.86 μM, P < 0.01)."
"There was no significant difference in the protein expression level of liver FMO3 among the groups"
". . . the gut microbiota composition was more sensitive to vitamin D3 supplementation than to a high-choline diet. Bacteroidetes, Proteobacteria, and Firmicutes were the main phyla in all groups at the phylum level, accounting for 90.65-93.57% of the gut microbiota community. A relatively higher abundance of Firmicutes was observed in the HC group (61.02%) than in either the C (47.82%) or HCD3 (48.65%) groups (P < 0.05), while the relative abundance of Bacteroidetes was much lower in the HC group (23.57%) than in either the C (41.71%) or HCD3 (39.34%) groups (P < 0.01; Figure 4(b)). The relative abundance of Proteobacteria among the groups was not significantly different. The Firmicutes/Bacteroidetes (F/B) ratio in the HC group (2.53 ± 0.19) was significantly larger than that in the C group (1.12 ± 0.06; P < 0.01), and the ratio was almost restored in the HCD3 group (1.21 ± 0.23; Figure 4(c))."
"To further identify correlations between plasma TMAO level and gut microbiota, Spearman correlation analysis was conducted. The results show that plasma TMAO levels were positively correlated with Lachnospiraceae_NK4A136_group (r = 0.78, P < 0.01) and negatively correlated with Bacteroides (r = −0.53, P < 0.05) in groups C and CD3 (Figures 6(a) and 6(b)). The plasma TMAO level was positively correlated with Lachnospiraceae_NK4A136_group (r = 0.46, P < 0.01) and negatively correlated with Bacteroides and Akkermansia (r = −0.66, P < 0.01; r = −0.25, P < 0.01) in groups HC and HCD3 (Figures 6(c)−6(e))."
"Our results indicate that the difference in liver FMO3 expression among different diet groups was not statistically significant. However, the TMAO level in the HC group was much higher than that in the HCD3 group. Therefore, vitamin D-induced decrease in plasma TMA and TMAO levels was not due to the regulation of FMO3 in the liver, and we hypothesized that the reduction in plasma TMA and TMAO levels can be attributed to the effect of changes in the composition of gut microbiota on the conversion of choline to TMA. Bennett et al. demonstrated that FMO3 expression is genetically regulated and that farnesoid X receptor (FXR) is a crucial regulator of the FMO3 level [26]. Liver FMO3 expression is gender-dependent in mice, and testosterone represses the expression of FMO3 in a male mouse liver, whereas females exhibit a high FMO3 level [27]. However, the regulated liver FMO3 expression and activity exact underlying mechanisms need to be further elucidated."
"The results of α-diversity analysis show that the ACE and Chao1 indices of group HC were much lower than those of group C, indicating that a high-choline diet decreased the richness of the gut microbiota community. However, vitamin D can improve the richness of gut microbiota caused by high-choline diet. In addition, the results of PCoA analysis demonstrate a significant separation between groups C and CD3 and between groups HC and HCD3, confirming that vitamin D supplementation can change the structure of gut microbiota. Our study found that Firmicutes was significantly more abundant and Bacteroidetes significantly less abundant in group HC in comparison with groups C and HCD3. This is consistent with the increased plasma TMA and TMAO levels and is in accordance with earlier reports showing that the ability to produce TMA is strong in Firmicutes but very weak in Bacteroidetes [28]."