Thanks for the explanation, which I forget often. But I don't understand why there are conflicting studies on Vitamin C's effect on iron absorption when taken with iron-rich meals. There must be some factor that's unaccounted for that explains the discrepancy in results.To be absorbed, dietary iron must be in the reduced ferrous form. Vitamin C reduces the ferric form of iron to the absorbable ferrous form. Heme iron is in the reduced ferrous (Fe2+) form. Plant iron is the oxidized ferric (Fe3+) form.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
-
By using this site you agree to the terms, rules, and privacy policy.
-
Charlie's Restoration Giveaway #2 (Entire Home EMF Mitigation & Protection Along With Personal Protection) - Click Here To Enter
-
Dear Carnivore Dieters, A Muscle Meat Only Diet is Extremely Healing Because it is a Low "vitamin A" Diet. This is Why it Works so Well...
Rest the rest of this post by clicking here
-
The Forum is transitioning to a subscription-based membership model - Click Here To Read
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Vitamin C
- Thread starter iLoveSugar
- Start date
-
- Tags
- supplemental vitamin c
InChristAlone
Member
Because heme iron is already super absorbable thus most people in this group need to be getting C to help offset the effects of heme iron. Vegeterians get way less iron because it is less absorbable than from liver or red meat. Not sure how much C is needed to help absorb plant iron or if it's even an issue since if you eat a lot of plants you likely don't need to worry about iron overload (unless you have hemochromatosis).Thanks for the explanation, which I forget often. But I don't understand why there are conflicting studies on Vitamin C's effect on iron absorption when taken with iron-rich meals. There must be some factor that's unaccounted for that explains the discrepancy in results.
alywest
Member
- Joined
- Apr 19, 2017
- Messages
- 1,028
@Janelle525 I just wanted to say thanks, and let you know that with all of the information you have provided on this forum I am convinced that extra Vitamin C (in addition to all the OJ I drink) is a good thing for me. Thanks for all of the interesting posts!
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
I've ordered Hormones and Resistance: Part 1 and Part 2 by Hans Selye a week ago and the book should have been delivered by now, but the seller just notified me that the order was canceled because as it turned out they only had Part 1. Anyways even if I'll order with amazon prime now I will get it on December 27th at best.Hans Selye did experiments with poisoning animals with mercury and showed that a given dose would cause death of the kidneys and such, but then he gave them the same amount of mercury plus vitamin C and they had no toxic effects at all.
And patience is not my strongest quality, not my quality at all
By any case any one of you guys @Travis @Amazoniac have it? If yes, could you please post Part 2 Chapter 7 Effects of Nonhormonal Factors upon Resistance page 578? Or I'll need ty get an electronic copy from google i guess
Last edited:
InChristAlone
Member
Glad I could help!@Janelle525 I just wanted to say thanks, and let you know that with all of the information you have provided on this forum I am convinced that extra Vitamin C (in addition to all the OJ I drink) is a good thing for me. Thanks for all of the interesting posts!
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
He has a 28‐page review article of the same title. Hans Selye is a good writer; here is an excerpt:I've ordered Hormones and Resistance: Part 1 and Part 2 by Hans Selye a week ago and the book should have been delivered by now, but the seller just notified me that the order was canceled because as it turned out they only had Part 1. Anyways even if I'll order with amazon prime now I will get it on December 27th at best.
And patience is not my strongest quality, not my quality at all
By any case any one of you guys @Travis @Amazoniac have it? If yes, could you please post Part 2 Chapter 7 Effects of Nonhormonal Factors upon Resistance page 578? Or I'll need try get an electronic copy from google i guess
'The term “dyskinesia” is used here to indicate all types of motor disturbances including simple prostration and tremor, which often appear in combination and are usually difficult to express more precisely. Only clear-cut convulsions (e.g., after digitoxin), anesthesia (e.g., after barbiturates or steroids), or muscular paralysis (e.g., after zoxazolamine) are specifically so identified.' —Selye
I haven't read any of his articles, but I know he has one on mast cells. I am reading about histamine in the brain right now: a less studied neurotransmitter produced by the tuberomammillary nucleus which sends projections throughout the brain similar to how the raphe nucleus distributes serotonin. There are also mast cells in the brain, and histamine brain concentrations can vary five‐fold among rats. Histamine no longer should be viewed as a forgotten step child of the field of neuropharmacology, as it is a powerful stimulant which has been expermimentally-shown to decrease memory retention and learning. Histamine is—perhaps along with pathologically low serotonin, μ-, and δ-exorphins—likely why celiac disease has been so correlated with schizophrenia (RR ≈ 4), autism correlated with immune activation and/or casein/wheat, and why general mental fog has been consistently observed by The Writer after eating the glutamine–proline‐rich seed storage proteins found in wheat (and oats, to a lesser extent). Casein has a few glutamine–proline repeats, but much less concentrated than grains. The allergens in nuts and beans represent lectins, and are of a different character altogether. It should be noted that the enzymatic proteolysis of gluten by prolypeptidases completely abrogates the interferon-γ spike seen after its ingestion, even among celiacs, proving conclusively that its the glutamine–proline bond which confers digestive resistance and subsequent immune response upon large peptide absorption. There is nothing inherently evil about these proteins besides their resistance to proteolysis, having bonds which pepsin and trypsin are incapable of breaking. The antibody formation is not particularly inimical; it is the release of interferon-γ which causes all the pathological downstream process seen after eating grains (and casein in some people) such as increased prostaglandin, nitric oxide, and histamine synthesis.
Last edited:
Mito
Member
- Joined
- Dec 10, 2016
- Messages
- 2,554
Maybe the design of the study? Some things decrease iron absorption for example calcium.Thanks for the explanation, which I forget often. But I don't understand why there are conflicting studies on Vitamin C's effect on iron absorption when taken with iron-rich meals. There must be some factor that's unaccounted for that explains the discrepancy in results.
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
Don't forget that it only works on inorganic iron, and does nothing to increase the absorption of iron already complexed with heme (as in beef). I think it's thought to increase absorption both by donating electrons to iron (forming Fe²⁺, and perhaps liberating it from certain affinity sites in food) and also perhaps by complexing with it. Iron is depicted below as covalently bonding to two ascorbates: http://en.chembase.cn/Server/MolImages/2E/53/2E53C9D3-8241-4168-A981-4F9ED33DEE5E.pngMaybe the design of the study? Some things decrease iron absorption for example calcium.
I don't know how well this compares with the citrate complex, which could also increase iron absorption. Citrate complexes very well with aluminum (stronger than pretty much anything) and iron (III) has the same charge and is close in diameter. Aluminum and iron are similar enough to displace eachother in the body (aluminum can be found carried by ferritin in the blood): https://www.sigmaaldrich.com/conten...cr_content/renditions/mfcd00050852-medium.png
Last edited:
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
You research sounds super interesting, hope you'll create a separate thread for your findings.He has a 28‐page review article of the same title. Hans Selye is a good writer; here is an excerpt:
'The term “dyskinesia” is used here to indicate all types of motor disturbances including simple prostration and tremor, which often appear in combination and are usually difficult to express more precisely. Only clear-cut convulsions (e.g., after digitoxin), anesthesia (e.g., after barbiturates or steroids), or muscular paralysis (e.g., after zoxazolamine) are specifically so identified.' —SelyeI haven't read any of his articles, but I know he has one on mast cells. I am reading about histamine in the brain right now: a less studied neurotransmitter produced by the tuberomammillary nucleus which sends projections throughout the brain similar to how the raphe nucleus distributes serotonin. There are also mast cells in the brain, and histamine brain concentrations can vary five‐fold among rats. Histamine no longer should be viewed as a forgotten step child of the field of neuropharmacology, as it is a powerful stimulant which has been expermimentally-shown to decrease memory retention and learning. Histamine is—perhaps along with pathologically low serotonin, μ-, and δ-exorphins—likely why celiac disease has been so correlated with schizophrenia (RR ≈ 4), autism correlated with immune activation and/or casein/wheat, and why general mental fog has been consistently observed by The Writer after eating the glutamine–proline‐rich seed storage proteins found in wheat (and oats, to a lesser extent). Casein has a few glutamine–proline repeats, but much less concentrated than grains. The allergens in nuts and beans represent lectins, and are of a different character altogether. It should be noted that the enzymatic proteolysis of gluten by prolypeptidases completely abrogates the interferon-γ spike seen after its ingestion, even among celiacs, proving conclusively that its the glutamine–proline bond which confers digestive resistance and subsequent immune response upon large peptide absorption. There is nothing inherently evil about these proteins besides their resistance to proteolysis, having bonds which pepsin and trypsin are incapable of breaking. The antibody formation is not particularly inimical; it is the release of interferon-γ which causes all the pathological downstream process seen after eating grains (and casein in some people) such as increased prostaglandin, nitric oxide, and histamine synthesis.
Too bad you don't have this book, Hans Selye talks about vitamin C on that page.
While we still on vitamin C thread: there are two natural products on the market with hight C content both derived from Acerola Cherry: frozen pure and freeze-dried powder. What do you think: which way of processing would retain more C in the final product?
L
lollipop
Guest
Good question. I am also curious.there are two natural products on the market with hight C content both derived from Acerola Cherry: frozen pure and freeze-dried powder. What do you think: which way of processing would retain more C in the final product?
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
Right?!I am also curious.
@lisaferraro just imagine: someone is actually living with @Travis, and s/he can ask him anything anytime! And he'll have an answer.
Lets just hope he will not get hit by a bus when walking drunk on his way home, lighting a cigarette and not looking both ways when crossing a road LOL
*and let's hope he has a sense of humor
L
lollipop
Guest
What an image! Made my day - lol, excuse me @Travis - really appreciating that sense of humor you have as mentioned by @noordinary; waaaay past the *hope* stageRight?!
@lisaferraro just imagine: someone is actually living with @Travis, and s/he can ask him anything anytime! And he'll have an answer.
Lets just hope he will not get hit by a bus when walking drunk on his way home, lighting a cigarette and not looking both ways when crossing a road LOL
*and let's hope he has a sense of humor
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
I would guess the freeze dried because it has less contact with water, something that could catalyze electron transfer—perhaps even while frozen.Good question. I am also curious.
'Acerola, also known as West Indian cherry, Barbados cherry or Cherry of Antilhas (Malpighia glabra L., Malpighia punicifolia L. or Malpighia emarginata DC.) is a fruit original from Antilhas which grows also in the northeast of South America and in Central America. It is round shaped, with diameter varying from 3 to 6 cm. [...] Its main appealing feature is the high Vitamin C content, which might vary from 1247.10 to 1845.79 mg/100 g [2], but it is also rich in other nutrients such as carotenes, thiamin, riboflavin, niacin, proteins, and mineral salts, mainly iron, calcium and phosphorus.' ―Marques
But after looking at Table 3 from Asami, I had nearly scrambled to delete what I had just typed. As you can see, there is more vitamin C in the frozen berries:
So you might wonder why I'm still typing? Well, it turns out that frozen berries continue to lose vitamin C at a constant rate. The table below was published in The Journal of the American Chemical Society, so it probably represents a fair depiction of reality.
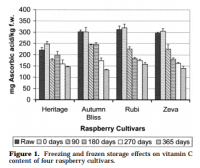 click to embiggen: Bar chart showing constant reduction of vitamin C of frozen berries upon cold storage.
click to embiggen: Bar chart showing constant reduction of vitamin C of frozen berries upon cold storage.This is a common finding but there is variation between fruits, and even between cultivars of the same fruit. Another study on frozen acreola purée has shown similar reductions:
'The total vitamin C content of acerola purées decreased after 150 days and then increased again at the end of the storage period for the clones evaluated, except II47/1 which remained constant. At harvest, clones BRS 238, BRS 236 and II 47/1 had the highest vitamin C contents (1,667, 1,664 and 1,653 mg. 100 FW g⁻¹, respectively, P> 0.05) and at the end of the trial, clones BRS 236 and 238 still had the highest values, 1,487 and 1,407 mg. 100 g⁻¹, respectively. The greatest total vitamin C loss was observed for clone II47/1, 39% over 10 months of storage and it could be explained by auto-oxidation, degradation by condensation due to high levels of anthocyanin or by oxidation by enzymes as ascorbate oxidase and peroxidase (Yahia et al., 2001). There is controversial data on the association between phenolics and vitamin C stability, for Miller and Rice-Evans (1997) reported that phenolics protected vitamin C from degradation, meanwhile Rosso and Mercadante (2007) implied that anthocyanin would condensate with ascorbic acid leading to a faster degradation of both compounds. Cultivars, agricultural practices, storage time and temperature also have great influence on vitamin C concentration during postharvest (Caris-Veyrat et al., 2004; Serpen et al., 2007; Garcia-Alonso et al., 2009).
Vitamin C consists of two forms, ascorbic acid and dehydroascorbic acid which are in a reversible equilibrium, although dehydroascorbic acid may also be irreversibly oxidized to 2,3-diketoglutonic acid. When peas were stored under freezing (-18°C) conditions for 12 months, there was a 91% loss of ascorbic acid and an estimated half-life of 3.12 months (Serpen et al., 2007). These authors reported that blanching increased the ascorbic acid half-life to 99.7 months and in order to retain vitamin C of processed vegetables, the key point is to limit the hydrolysis of dehydroascorbic acid into 2,3-diketoglutonic acid, thus the loss of vitamin C during storage depends on the balance of oxidation and reduction capacities. In spite of the loss during storage, the total vitamin C contents of the acerola purées studied were still much higher than for most commercialized fruit as apple cv. Gala pulp (not detected), tomato (17.5 mg. FW 100 g⁻¹), banana (18.6 mg. FW 100 g⁻¹), pineapple (22 mg. FW 100 g⁻¹), star fruit (37.4 mg. FW 100 g⁻¹), red guava pulp (56 mg. FW 100 g⁻¹), lemon (74 mg. FW 100 g⁻¹), orange pulp (83 mg. FW 100 g⁻¹) and persimmon (210 mg. FW 100 g⁻¹) (Lee and Kader, 2000; Caris-Veyrat et al., 2004; Hassimotto et al., 2005).' ―Oliveira
Freeze–dried acreola berries start out with an immediate reduction of vitamin C content:
And a determination of two year old camu‐camu powder (freeze–dried) implies that it could be stable. After adjusting the units (or shifting the decimal point), the freeze–dried camu‐camu powder has over half the vitamin C than immediately freeze–dried acreola.
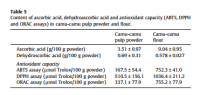 click to embiggen: Table showing vitamin C content in freeze–dried powder aged approximately two years.
click to embiggen: Table showing vitamin C content in freeze–dried powder aged approximately two years.But it could actually have more to start with. The camu‐camu berry has quite a bit. The low end of the range reported here represents the upper range for acreola reported above (after adjusting for disparate units):
'Camu-camu is a native Amazonian bush from the Myrtaceae family. Its fruits are round berries having an average diameter of 2.5 cm; its pulp is pink, while its skin is green when immature and changes during the ripening process from green to red-purple due to the presence of anthocyanins (Zanatta & Mercadante, 2005). Camu-camu is appreciated for its high content of ascorbic acid, which varies from 1.9 to 2.3 g/100 g fresh matter depending on the maturity stage (Chirinos, Galarza, Betalleluz-Pallardel, Pedresch, & Campos, 2010). Compared to other fruits, camu-camu is considered one of the richest sources of vitamin C, with a higher content than acerola (Rufino et al., 2010).' ―Fracassetti
Without knowing the age, it's hard to say; and there isn't much data on the stability of freeze–dried berry powder over time. It's obvious that freeze–dried berries lose more vitamin C initially, but frozen berries could end up lower after a year or two. Freeze–drying represents an initial ~10–66% loss (depending on berry maturity and species) but frozen berries lose ~50% after one year.
If freeze–dried berries are cheaper (they're cheaper to ship, certainly), then it might be better to just eat twice as much of the freeze–dried berry. If I didn't eat mostly fruit as it is, I would perhaps go with the freeze–dried berry powders and keep them in the freezer. Besides having ascorbate they have carotenes (protoretinoids) and minerals. Some polyphenols can also be beneficial.
Freeze–dried berry powder is hygroscopic; it absorbs water from the air; this increases with increasing temperature and humidity. Adsorbed water would be expected to increase the rate of ascorbate oxidation. For maximum vitamin C stability, berry powder should be kept dry until use.
Although citrus fruits start out with a lower concentration than these exotic berries, this difference is attenuated by the losses incurred through freezing and freeze–drying. Limes store nearly just as well as freeze–dried berry powder (just ask the nearest British sailor for confirmation of this).
Marques, Luanda G. "Freeze-drying of acerola (Malpighia glabra L.)." Chemical Engineering and Processing: Process Intensification (2007)
Ribeiro, Luciana C. "Hygroscopic behavior of lyophilized acerola pulp powder." Revista Brasileira de Engenharia Agrícola e Ambiental (2016)
Oliveira, Luciana S. "The influence of processing and long-term storage on the antioxidant metabolism of acerola (Malpighia emarginata) purée." Brazilian Journal of Plant Physiology (2011)
Fracassetti, Daniela. "Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit." Food Chemistry (2013)
de Ancos, Begoña. "Ellagic acid, vitamin C, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit." Journal of agricultural and food chemistry (2000)
Asami, Danny K. "Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable..." Journal of agricultural and food chemistry (2003)
Ribeiro, Luciana C. "Hygroscopic behavior of lyophilized acerola pulp powder." Revista Brasileira de Engenharia Agrícola e Ambiental (2016)
Oliveira, Luciana S. "The influence of processing and long-term storage on the antioxidant metabolism of acerola (Malpighia emarginata) purée." Brazilian Journal of Plant Physiology (2011)
Fracassetti, Daniela. "Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit." Food Chemistry (2013)
de Ancos, Begoña. "Ellagic acid, vitamin C, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit." Journal of agricultural and food chemistry (2000)
Asami, Danny K. "Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable..." Journal of agricultural and food chemistry (2003)
Last edited:
Fractality
Member
- Joined
- Jan 23, 2016
- Messages
- 772
Does anyone recommend a particular source for acerola cherry fruit and/or powder?
L
lollipop
Guest
Very helpful! Thank you @Travis Interesting about limes. I have been drawn to making a glass of fresh lemonade each morning au lieu de drinking orange juice for the last few months - maybe the available C is why...I would guess the freeze dried because it has less contact with water, something that could catalyze electron transfer—perhaps even while frozen.
'Acerola, also known as West Indian cherry, Barbados cherry or Cherry of Antilhas (Malpighia glabra L., Malpighia punicifolia L. or Malpighia emarginata DC.) is a fruit original from Antilhas which grows also in the northeast of South America and in Central America. It is round shaped, with diameter varying from 3 to 6 cm. [...] Its main appealing feature is the high Vitamin C content, which might vary from 1247.10 to 1845.79 mg/100 g [2], but it is also rich in other nutrients such as carotenes, thiamin, riboflavin, niacin, proteins, and mineral salts, mainly iron, calcium and phosphorus.' ―Marques
But after looking at Table 3 from Asami, I had nearly scrambled to delete what I had just typed. As you can see, there is more vitamin C in the frozen berries:
View attachment 7668 click to embiggen: Table showing frozen berries with more vitamin C than freeze–dried berries.
So you might wonder why I'm still typing? Well, it turns out that frozen berries continue to lose vitamin C at a constant rate. The table below was published in The Journal of the American Chemical Society, so it probably represents a fair depiction of reality.
View attachment 7669 click to embiggen: Bar chart showing constant reduction of vitamin C of frozen berries upon cold storage.
This is a common finding but there is variation between fruits, and even between cultivars of the same fruit. Another study on frozen acreola purée has shown similar reductions:
'The total vitamin C content of acerola purées decreased after 150 days and then increased again at the end of the storage period for the clones evaluated, except II47/1 which remained constant. At harvest, clones BRS 238, BRS 236 and II 47/1 had the highest vitamin C contents (1,667, 1,664 and 1,653 mg. 100 FW g⁻¹, respectively, P> 0.05) and at the end of the trial, clones BRS 236 and 238 still had the highest values, 1,487 and 1,407 mg. 100 g⁻¹, respectively. The greatest total vitamin C loss was observed for clone II47/1, 39% over 10 months of storage and it could be explained by auto-oxidation, degradation by condensation due to high levels of anthocyanin or by oxidation by enzymes as ascorbate oxidase and peroxidase (Yahia et al., 2001). There is controversial data on the association between phenolics and vitamin C stability, for Miller and Rice-Evans (1997) reported that phenolics protected vitamin C from degradation, meanwhile Rosso and Mercadante (2007) implied that anthocyanin would condensate with ascorbic acid leading to a faster degradation of both compounds. Cultivars, agricultural practices, storage time and temperature also have great influence on vitamin C concentration during postharvest (Caris-Veyrat et al., 2004; Serpen et al., 2007; Garcia-Alonso et al., 2009).
Vitamin C consists of two forms, ascorbic acid and dehydroascorbic acid which are in a reversible equilibrium, although dehydroascorbic acid may also be irreversibly oxidized to 2,3-diketoglutonic acid. When peas were stored under freezing (-18°C) conditions for 12 months, there was a 91% loss of ascorbic acid and an estimated half-life of 3.12 months (Serpen et al., 2007). These authors reported that blanching increased the ascorbic acid half-life to 99.7 months and in order to retain vitamin C of processed vegetables, the key point is to limit the hydrolysis of dehydroascorbic acid into 2,3-diketoglutonic acid, thus the loss of vitamin C during storage depends on the balance of oxidation and reduction capacities. In spite of the loss during storage, the total vitamin C contents of the acerola purées studied were still much higher than for most commercialized fruit as apple cv. Gala pulp (not detected), tomato (17.5 mg. FW 100 g⁻¹), banana (18.6 mg. FW 100 g⁻¹), pineapple (22 mg. FW 100 g⁻¹), star fruit (37.4 mg. FW 100 g⁻¹), red guava pulp (56 mg. FW 100 g⁻¹), lemon (74 mg. FW 100 g⁻¹), orange pulp (83 mg. FW 100 g⁻¹) and persimmon (210 mg. FW 100 g⁻¹) (Lee and Kader, 2000; Caris-Veyrat et al., 2004; Hassimotto et al., 2005).' ―Oliveira
Freeze–dried acreola berries start out with an immediate reduction of vitamin C content:
View attachment 7670 click to embiggen: Table showing reduction in vitamin C content upon freeze–drying (note the disparate units between the first column and the other two).
And a determination of two year old camu‐camu powder (freeze–dried) implies that it could be stable. After adjusting the units (or shifting the decimal point), the freeze–dried camu‐camu powder has over half the vitamin C than immediately freeze–dried acreola.
View attachment 7671 click to embiggen: Table showing vitamin C content in freeze–dried powder aged approximately two years.
But it could actually have more to start with. The camu‐camu berry has quite a bit. The low end of the range reported here represents the upper range for acreola reported above (after adjusting for disparate units):
'Camu-camu is a native Amazonian bush from the Myrtaceae family. Its fruits are round berries having an average diameter of 2.5 cm; its pulp is pink, while its skin is green when immature and changes during the ripening process from green to red-purple due to the presence of anthocyanins (Zanatta & Mercadante, 2005). Camu-camu is appreciated for its high content of ascorbic acid, which varies from 1.9 to 2.3 g/100 g fresh matter depending on the maturity stage (Chirinos, Galarza, Betalleluz-Pallardel, Pedresch, & Campos, 2010). Compared to other fruits, camu-camu is considered one of the richest sources of vitamin C, with a higher content than acerola (Rufino et al., 2010).' ―Fracassetti
Without knowing the age, it's hard to say; and there isn't much data on the stability of freeze–dried berry powder over time. It's obvious that freeze–dried berries lose more vitamin C initially, but frozen berries could end up lower after a year or two. Freeze–drying represents an initial ~10–66% loss (depending on berry maturity and species) but frozen berries lose ~50% after one year.
If freeze–dried berries are cheaper (they're cheaper to ship, certainly), then it might be better to just eat twice as much of the freeze–dried berry. If I didn't eat mostly fruit as it is, I would perhaps go with the freeze–dried berry powders and keep them in the freezer. Besides having ascorbate they have carotenes (protoretinoids) and minerals. Some polyphenols can also be beneficial.
Freeze–dried berry powder is hygroscopic; it absorbs water from the air; this increases with increasing temperature and humidity. Adsorbed water would be expected to increase the rate of ascorbate oxidation. For maximum vitamin C stability, berry powder should be kept dry until use.
Although citrus fruits start out with a lower concentration than these exotic berries, this difference is attenuated by the losses incurred through freezing and freeze–drying. Limes store nearly just as well as freeze–dried berry powder (just ask the nearest British sailor for confirmation of this).
Marques, Luanda G. "Freeze-drying of acerola (Malpighia glabra L.)." Chemical Engineering and Processing: Process Intensification (2007)
Ribeiro, Luciana C. "Hygroscopic behavior of lyophilized acerola pulp powder." Revista Brasileira de Engenharia Agrícola e Ambiental (2016)
Oliveira, Luciana S. "The influence of processing and long-term storage on the antioxidant metabolism of acerola (Malpighia emarginata) purée." Brazilian Journal of Plant Physiology (2011)
Fracassetti, Daniela. "Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit." Food Chemistry (2013)
de Ancos, Begoña. "Ellagic acid, vitamin C, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit." Journal of agricultural and food chemistry (2000)
Asami, Danny K. "Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable..." Journal of agricultural and food chemistry (2003)
Amazoniac
Member
How do they guarantee a standardized content? I'm asking because these fruits that are very high in vit C vary a lot: some taste really sweet, whereas some are bitter; some have a dark wine color while others are yellow or even unripe green. I'm certain that it will vary depending on the batch unless they do something to minimize the variation, such as freezing and mixing, I don't know..
InChristAlone
Member
I personally would not trust a whole food supp to get enough C. Use fresh ripe fruit. I doubt I was getting near enough C from bottled OJ, it's so processed it was probably all oxidized. Peat says oxidized is fine, but obviously in my case it wasn't enough.
Last edited:
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
Yeah. What surprised me was: The green acreola berries were reduced in vitamin C content at a far greater ratio upon freeze–drying. Also surprising was the variation between strains—an effect attributed to other redox‐actice molecules present, such as the polyphenols. The camu‐camu berry not only has a name which reminds a person of the hypothetical camel–emu chimera (and two of them together, as conjoined twins), but is also higher in vitamin C that the acreola (a.k.a. West Indian cherry, Barbados cherry, Cherry of Antilhas, and Malpighia emarginata).How do they guarantee a standardized content? I'm asking because these fruits that are very high in vit C vary a lot: some taste really sweet, whereas some are bitter; some have a dark wine color while others are yellow or even unripe green. I'm certain that it will vary depending on the batch unless they do something to minimize the variation, such as freezing and mixing, I don't know..
I think Janelle is probably right about the orange juice. I think they might add some after pasteurization, but since this is not even frozen you would expect this to decay at an even greater rate—making the value printed on the label representing a deceptive inflation of the actual value.
Last edited:
Amazoniac
Member
I would opt for camu-camu as well, because if you happen to receive a bag with low content, the chances of still being higher than other fruits is greater.Yeah. What surprised me was: The green acreola berries were reduced in vitamin C content at a far greater ratio upon freeze–drying. Also surprising was the variation between strains—an effect attributed to other redox‐actice molecules present, such as the polyphenols. The camu‐camu berry not only has a name which reminds a person of the hypothetical camel–emu chimera (and two of them together, as conjoined twins), but is also higher in vitamin C that the acreola (a.k.a. West Indian cherry, Barbados cherry, Cherry of Antilhas, and Malpighia emarginata).
I think Janelle is probably right about the orange juice, but I think they might add some after pasteurization. But since this is not even frozen, you would expect this to decay at an alarming rate—making the value printed on the label representative of a deceptive inflation of the actual value. I am sure there's more information on the temporal stability of commercial orange juice than that on freeze–dried acreola cherry, so an answer is probably on a click away.
The vit C content of acerolas where I live reinforce my point. They can be lower than 950mg/100g. I suspect you can find even lower than that. On the other hand, there are ones with more than 2800mg/100g.
Moral of the story is: you can't trust the system.. maan.
Last edited:
sladerunner69
Member
I doubt that we need at least 3g to maintain health, my guess would be around that range.
Do you think it's possible that there are vit C supplements on the market providing part of their product as dummy rounds and therefore needing higher dosages to reach the desired effect? Or perhaps there are enough oxidants from contamination that part of its reducing nature is lost before abortI mean, absorption? I linked Ray's interview because I can't think for myself and I don't trust my experience, but I don't represent the forum; if anything, I'm just one of pboy's pawns.
What about the studies of 50grams+ vitC administerred intravenously? that was supposedly of high purity, low risk for contam (although perhaps not). Doses in this fashion the benefits seemed to accrue as the dose moved upward...
Furthermore orange juice seems to be a superior source of vitamin C because the nutrient doesn't evaporate to the air as easily as many other juices...
EMF Mitigation - Flush Niacin - Big 5 Minerals
Similar threads
- Replies
- 36
- Views
- 20K
- Replies
- 5
- Views
- 4K
- Replies
- 63
- Views
- 22K

