I find Internet Archive: Digital Library of Free Books, Movies, Music & Wayback Machine website also good when searching for certain books.How do you find these things?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
-
By using this site you agree to the terms, rules, and privacy policy.
-
Charlie's Restoration Giveaway #2 (Entire Home EMF Mitigation & Protection Along With Personal Protection) - Click Here To Enter
-
Dear Carnivore Dieters, A Muscle Meat Only Diet is Extremely Healing Because it is a Low "vitamin A" Diet. This is Why it Works so Well...
Rest the rest of this post by clicking here
-
The Forum is transitioning to a subscription-based membership model - Click Here To Read
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
The Travis Corner
- Thread starter Amazoniac
- Start date
Mito
Member
- Joined
- Dec 10, 2016
- Messages
- 2,554
That’s unusual, there are studies that estimate 85% of the population has some combination of homozygous/heterozygous of either or both of the C677T mutation or A1298C mutation of MTHFR.i had DNA analysis I don't have any mutations (including MTFR)
The drop you saw in homocysteine could be a result of more creatine and choline in your diet. Do you think that is the case?
About 40-45% of methylation is used to make creatine, and another 40-45% is used to make phosphatidylcholine. So if either creatine or choline is low in your diet then you have to make it which requires methylation which generates homocysteine.
Last edited:
Koveras
Member
- Joined
- Dec 17, 2015
- Messages
- 720
The decarboxylated carbon skeleton of leucine is reminiscent of isoprene—the cholesterol building block monomer—and studies using ¹⁴C‐leucine have shown it to be incorporated directly into the steroid ring. The body can apparently bypass many steps of cholesterol biosynthesis by using spare leucine directly.
That's interesting because in digging further why whole eggs stimulated protein synthesis to a greater degree then egg whites I found this
"We are seeing greater movement of mTORC1 to the cell periphery (the hub for dietary amino acids into the muscle) in the whole egg condition vs. egg white condition (preliminary/unpublished results). "
[Digging...]
“In his own laboratory, Roberto recently discovered that mTORC1 senses cholesterol through a pathway that also controls its translocation to lysosomes (77), extending the range of nutrients these organelles sense beyond the amino acids and glucose we have focused on.”
Sabatini, D. M. (2017). Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proc Natl Acad Sci U S A, 114(45), 11818-11825. doi:10.1073/pnas.1716173114
["77"]
Castellano, B. M., Thelen, A. M., Moldavski, O., Feltes, M., van der Welle, R. E., Mydock-McGrane, L., . . . Zoncu, R. (2017). Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science, 355(6331), 1306-1311. doi:10.1126/science.aag1417
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
This is exactly how I perceived it after thinking about how leucine could do anything. I had taken a look at leucine − CO₂ and saw it as a 'saturated isoprene,' which could perhaps be used for cholesterol and heme synthesis. This has actually been shown to be true, and we do have a leucine sensor. Leucine first appears to be decarboxylated to isopenylamine, then hyrolyzed to isoamyl alcohol. If this is then phosphorylated, you have isopentenyl pyrophosphate—circumventing the entire mevalonate pathway. This must occur, to a degree, since ¹⁴C‐leucine has been shown incorporated into cholesterol (see Pellagra thread).That's interesting because in digging further why whole eggs stimulated protein synthesis to a greater degree then egg whites I found this
"We are seeing greater movement of mTORC1 to the cell periphery (the hub for dietary amino acids into the muscle) in the whole egg condition vs. egg white condition (preliminary/unpublished results). "
[Digging...]
“In his own laboratory, Roberto recently discovered that mTORC1 senses cholesterol through a pathway that also controls its translocation to lysosomes (77), extending the range of nutrients these organelles sense beyond the amino acids and glucose we have focused on.”
Sabatini, D. M. (2017). Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proc Natl Acad Sci U S A, 114(45), 11818-11825. doi:10.1073/pnas.1716173114
["77"]
Castellano, B. M., Thelen, A. M., Moldavski, O., Feltes, M., van der Welle, R. E., Mydock-McGrane, L., . . . Zoncu, R. (2017). Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science, 355(6331), 1306-1311. doi:10.1126/science.aag1417
Here we go:
Rosenthal, J. "Metabolic fate of leucine: a significant sterol precursor in adipose tissue and muscle." American Journal of Physiology--Legacy Content (1974)
'With either leucine concentration, hydrocarbons and squalene made up 19% of total sterols, 55 % of the newly synthesized NSL. Approximately 63 % of the sterols were cholesterol.' ―Rosenthal
'Incorporation of leucine into sterols was 10 times greater than for isoleucine, while valine was poorly utilized when compared to either leucine or isoleucine (Table 3).' ―Rosenthal
'The role of leucine as a precursor of body cholesterol in the rat was first demonstrated by Bloch in 1944 (4). More recently, incorporation of leucine-³H into serum cholesterol in both rats and humans has been reported (26, 27). Miettinen and Penttila (26, 27) have suggested that muscle may be the major extrahepatic site where leucine is converted into cholesterol prior to release into the circulation. However, the data reported here (Table 5) indicate that the adipose organ may be just as active a site of sterol synthesis as muscle. Obesity in humans is associated with an increased rate of cholesterol production (29) which can be decreased by weight reduction (25). Since the proportion of body weight represented by fat tissue in obesity may greatly exceed the normal 15% (21, 28), the adipose organ must be viewed as a potential source of this cholesterol. The role of leucine as a precursor must now be considered in light of the preliminary data in Table 9, which suggest that human adipose tissue can utilize leucine for sterol synthesis and the fact that plasma leucine and insulin levels are elevated in obese man (11). Whether leucine conversion to sterols contributes significantly to the increased cholesterol production rate observed in obese man awaits further experimentation.' ―Rosenthal
'Incorporation of leucine into sterols was 10 times greater than for isoleucine, while valine was poorly utilized when compared to either leucine or isoleucine (Table 3).' ―Rosenthal
'The role of leucine as a precursor of body cholesterol in the rat was first demonstrated by Bloch in 1944 (4). More recently, incorporation of leucine-³H into serum cholesterol in both rats and humans has been reported (26, 27). Miettinen and Penttila (26, 27) have suggested that muscle may be the major extrahepatic site where leucine is converted into cholesterol prior to release into the circulation. However, the data reported here (Table 5) indicate that the adipose organ may be just as active a site of sterol synthesis as muscle. Obesity in humans is associated with an increased rate of cholesterol production (29) which can be decreased by weight reduction (25). Since the proportion of body weight represented by fat tissue in obesity may greatly exceed the normal 15% (21, 28), the adipose organ must be viewed as a potential source of this cholesterol. The role of leucine as a precursor must now be considered in light of the preliminary data in Table 9, which suggest that human adipose tissue can utilize leucine for sterol synthesis and the fact that plasma leucine and insulin levels are elevated in obese man (11). Whether leucine conversion to sterols contributes significantly to the increased cholesterol production rate observed in obese man awaits further experimentation.' ―Rosenthal
Koveras
Member
- Joined
- Dec 17, 2015
- Messages
- 720
'The role of leucine as a precursor of body cholesterol in the rat was first demonstrated by Bloch in 1944 (4). More recently, incorporation of leucine-³H into serum cholesterol in both rats and humans has been reported (26, 27). Miettinen and Penttila (26, 27) have suggested that muscle may be the major extrahepatic site where leucine is converted into cholesterol prior to release into the circulation. However, the data reported here (Table 5) indicate that the adipose organ may be just as active a site of sterol synthesis as muscle. Obesity in humans is associated with an increased rate of cholesterol production (29) which can be decreased by weight reduction (25). Since the proportion of body weight represented by fat tissue in obesity may greatly exceed the normal 15% (21, 28), the adipose organ must be viewed as a potential source of this cholesterol. The role of leucine as a precursor must now be considered in light of the preliminary data in Table 9, which suggest that human adipose tissue can utilize leucine for sterol synthesis and the fact that plasma leucine and insulin levels are elevated in obese man (11). Whether leucine conversion to sterols contributes significantly to the increased cholesterol production rate observed in obese man awaits further experimentation.' ―Rosenthal
Reminded me of this
J Physiol. 2017 Dec 19. doi: 10.1113/JP275075. [Epub ahead of print]
Restoration of metabolic health by decreased consumption of branched-chain amino acids.
Cummings NE1,2,3, Williams EM1,2, Kasza I4, Konon EN1,2, Schaid MD1,2,5, Schmidt BA1,2, Poudel C1,2, Sherman DS1,2, Yu D1,2,6, Arriola Apelo SI1,2,7, Cottrell SE1,2,8, Geiger G1,2, Barnes ME1,2, Wisinski JA1,2, Fenske RJ1,2,5, Matkowskyj KA2,9,10, Kimple ME1,2,3,5,11, Alexander CM4, Merrins MJ1,2,12, Lamming DW1,2,3,5,6,9.
KEY POINTS:
We recently found that feeding healthy mice a diet with reduced levels of branched-chain amino acids (BCAAs), which are associated with insulin resistance in both humans and rodents, modestly improves glucose tolerance and slows fat mass gain. In the present study, we show that a reduced BCAA diet promotes rapid fat mass loss without calorie restriction in obese mice. Selective reduction of dietary BCAAs also restores glucose tolerance and insulin sensitivity to obese mice, even as they continue to consume a high-fat, high-sugar diet. A low BCAA diet transiently induces FGF21 (fibroblast growth factor 21) and increases energy expenditure. We suggest that dietary protein quality (i.e. the precise macronutrient composition of dietary protein) may impact the effectiveness of weight loss diets.
ABSTRACT:
Obesity and diabetes are increasing problems around the world, and although even moderate weight loss can improve metabolic health, reduced calorie diets are notoriously difficult to sustain. Branched-chain amino acids (BCAAs; leucine, isoleucine and valine) are elevated in the blood of obese, insulin-resistant humans and rodents. We recently demonstrated that specifically reducing dietary levels of BCAAs has beneficial effects on the metabolic health of young, growing mice, improving glucose tolerance and modestly slowing fat mass gain. In the present study, we examine the hypothesis that reducing dietary BCAAs will promote weight loss, reduce adiposity, and improve blood glucose control in diet-induced obese mice with pre-existing metabolic syndrome. We find that specifically reducing dietary BCAAs rapidly reverses diet-induced obesity and improves glucoregulatory control in diet-induced obese mice. Most dramatically, mice eating an otherwise unhealthy high-calorie, high-sugar Western diet with reduced levels of BCAAs lost weight and fat mass rapidly until regaining a normal weight. Importantly, this normalization of weight was mediated not by caloric restriction or increased activity, but by increased energy expenditure, and was accompanied by a transient induction of the energy balance regulating hormone FGF21 (fibroblast growth factor 21). Consumption of a Western diet reduced in BCAAs was also accompanied by a dramatic improvement in glucose tolerance and insulin resistance. Our results link dietary BCAAs with the regulation of metabolic health and energy balance in obese animals, and suggest that specifically reducing dietary BCAAs may represent a highly translatable option for the treatment of obesity and insulin resistance.
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
I am quite a subject lol, i don't have some pretty common things, like for example I'm Rh negative.That’s unusual, there are studies that estimate 85% of the population has some combination of homozygous/heterozygous of either or both of the C677T mutation or A1298C mutation of MTHFR.
The drop you saw in homocysteine could be a result of more creatine and choline in your diet. Do you think that is the case?
About 40-45% of methylation is used to make creatine, and another 40-45% is used to make phosphatidylcholine. So if either creatine or choline is low in your diet then you have to make it which requires methylation which generates homocysteine.
But you are actually right (from my DNA report):
I have heterozygous A1298C mutation.
I found this:
MTHFR A1298C Mutation: Some Information on A1298C MTHFR Mutations - MTHFR.Net
My homocysteine dropped in half in one month after adding butter and egg yolks to my diet (back then i had hard time even thinking about meats, so i didn't eat meat, but i craved butter like crazy last two months of being vegan, i literally was dreaming about eating butter by a tablespoon, it was that ridiculous)
Mito
Member
- Joined
- Dec 10, 2016
- Messages
- 2,554
Yes that one has the least effect of them all.I have heterozygous A1298C mutation.
If you’re heterozygous for A1298C and you don’t have any C677T mutation, you have a 17% decrease in MTHFR activity. If you’re homozygous, you have a 39% decrease. Granted, these are averages taken from a study, and it’s 17% give or take, 39% give or take. But it gives you a general idea of the magnitude of the effect.
The C677T mutation is stronger than the A1298C mutation. For heterozygous, you have a 33% decrease instead of a 17% decrease. For homozygous, you have a 75% decrease instead of a 39% decrease. So to be homozygous for A1298C is only slightly worse than to be heterozygous for C677T. If you are compound heterozygous, meaning you have one A1298C mutation and one C677T mutation, you have a 53%, on average, give or take, decrease of MTHFR activity. You cut it a little bit more than in half.
But another way to look at this is this is a continuum. There’s no categorical difference between someone who’s homozygous for C677T and someone who’s heterozygous for A1298C. The effect is 4-5 times greater, but it’s not categorical. It’s continuous. And then you have to consider, well, the average homocysteine might not be different in one group versus another, but that doesn’t mean that one of those people doesn’t have higher homocysteine, or some of those people. And it doesn’t mean that those people if put on a really good diet versus a really bad diet aren’t gonna see that effect come to life.
https://chrismasterjohnphd.com/2017/08/12/living-with-mthfr/
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
The drop you saw in homocysteine could be a result of more creatine and choline in your diet. Do you think that is the case?
From my notes:The Pearson correlation coefficient of cobalamin vs homocysteine in vegans was measured to be only −.42 (Krajčovičová-Kudláčková, 2000).
In my case twice increase in B12 serum concentration correlated with twice decrease in Homocysteine.
Mito
Member
- Joined
- Dec 10, 2016
- Messages
- 2,554
Do you have any of these?In my case twice increase in B12 serum concentration correlated with twice decrease in Homocysteine.
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
B6 was not measured with any of my blood work, but i suspect that the levels should have been normal or high, considering that even kale is a better source or B6 than egg yolk.Pyroxidal (B₆) is measured because it is the cofactor for the enzymes which convert homocysteine into the much safer cysteine. The vegans consumed more niacin (B₃) which removes methyl groups. These methylation trends appear somewhat predictable, so keeping the homocysteine/methionine ratio to a minimum is mostly a matter of consuming the proper vitamins: cobalamin (B₁₂), pyroxidal (B₆), and folate (B₉)—the ones with subscripts divisible by three. Also thought to play a role are methyl donors (i.e. betaine; choline; creatine; methylene blue).
Folate was high.
So it brings us to B12:
source: Genetics of homocysteine metabolism and associated disorders
B12 is a cofactor in remethylation with folate in all tissues, only reaction with betain (restricted to the liver) is independent of B12
It's not one of B12, B6 and Folate, it's all of them together that are required.
For me the point is (yes no paradox):
there is a nonlinear relationship between methionine and homocysteine:
you may have high homocysteine consuming very little methionine
and
you may have low homocysteine consuming a lot of methionine
and
that depends on several thing
mostly on B12,B6 and Folate.
Where that places vegans who don't supplement B vitamins and animal protein eaters who may not even supplement B vitamins, but eat B vitamins reach food, in my case the latter was associated with lower and eventually even lower homocysteine levels.
Ideally would be eating food low in methionine and high in B6, B9 and B12:
Foods highest in Vitamin B6 and Folate and Vitamin B12, and lowest in Methionine
ok, lets be honest this Search sucks.
But does place Lamb liver (Crude liver extract was part of Gerson theraphy) right after Cereals Bahahhaha
As for the Linoleic acid, here is an unfair comparison (i did eat about a pound of leaves a day while being vegan though):
according to SELF Nutrition Data | Food Facts, Information & Calorie Calculator
which we can't fully trust obviously.
Last edited:
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
Yep:
But I don't think that was the case for me (that i wasn't able to regenerate Methyl-B12)
because between August 2014 and July 2015 I was vegan with no exogenous source of B12 and my serum B12 level changed ever so slightly from 198 to 189 over a course of almost a year.
Last edited:
Mito
Member
- Joined
- Dec 10, 2016
- Messages
- 2,554
Yes but it’s not necessarily mostly B12, B6, and folate (in your case it probably was) because you can also completely bypass the folate/b12 homocysteine recycling path and use only the BHMT path which is mostly about choline which converts to betaine. You can also probably significantly drop homocysteine without either the b vitamin path or BHMT paths by adding lots of creatine to your diet.For me the point is (yes no paradox):
there is a nonlinear relationship between methionine and homocysteine:
you may have high homocysteine consuming very little methionine
and
you may have low homocysteine consuming a lot of methionine
and
that depends on several thing
mostly on B12,B6 and Folate.
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
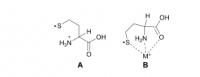
Even though homocysteine is highly correlated with lipid peroxidation in the cerebospinal fluid (r=.92), it needs help from nitric oxide to be harmful.
The reason why homocysteine, and not cysteine, is so correlated with lipid peroxidation is that it's capable of forming a stable free radical. Once a sulfur radical is formed (unpaired electron), it can transfer to the α-carbon. Cysteine can indeed form a radical on its sulfur atom, but its tail is not long enough for transfer this to the α-carbon.
This radical electron shift has been shown to be catalyzed by K⁺, Na⁺, and Li⁺ in the gas phase:
You might think that anything which brings the sulfur radical in proximity to the amide can potentially catalyze the radical transfer to the α-carbon.
But for the initial thiyl radical (S·) to form in the first place, nitric oxide is needed. This binds with the thiol and is then cleaved homolytically, putting a radical unpaired electron on both species:
This radical electron (·) can shift to the α-carbon, an event catalyzed by sodium and potassium. This forms the stable free radical which can cross the blood–brain barrier, being nearly indistinguishable from normal homocysteine. Like a Trojan Horse it gains access to the highly-unsaturated brain lipids causing a lipid peroxidation cascade, ostensibly why you have a Pearson coefficient of .92 between cerebospinal homocysteine and hydroxynonenal in same fluid. Hydroxynonenal is a lipid peroxidation product capable of crosslinking proteins.
So I view homocysteine as a molecule capable of transporting radicals formed in the periphery into the brain. But for this to occur, the initial sulfur radical must form through an interaction with nitric oxide—another reason to avoid nitrates.
And like cyclooxygenase and phospholipase A₂, the enzyme nitric oxide synthase is also inducible by cytokines (iNOS). This enzyme cleaves nitric oxide off of arginine forming citrulline, and eventually ornithine upon further reactions.
Although it's common to measure plasma homocysteine, it is unheard of to determine the plasma concentration of the radical subspecies. This would be interesting to look into. The radical species is generally given credit for homocysteine dementia, although homocysteine can also form a cyclic thiolactone as well as modifying proteins directly (homocysteinylation).
The reason why homocysteine, and not cysteine, is so correlated with lipid peroxidation is that it's capable of forming a stable free radical. Once a sulfur radical is formed (unpaired electron), it can transfer to the α-carbon. Cysteine can indeed form a radical on its sulfur atom, but its tail is not long enough for transfer this to the α-carbon.
'The sulfur-based radical of homocysteine is of interest because of its ability to migrate from the sulfur atom to the α-carbon position much more easily (about 10 times faster in solution at pH 10) than in the cysteine radical.' ―Osburn
This radical electron shift has been shown to be catalyzed by K⁺, Na⁺, and Li⁺ in the gas phase:
You might think that anything which brings the sulfur radical in proximity to the amide can potentially catalyze the radical transfer to the α-carbon.
'In this work we show that metal ion complexation to the Hcy thiyl radical induces facile HAT. To our knowledge, this is the first example of a gas-phase study where conversion of an ·S‐radical into a Cα‐radical via hydrogen atom transfer occurs within a single amino acid residue.' ―Lesslie
But for the initial thiyl radical (S·) to form in the first place, nitric oxide is needed. This binds with the thiol and is then cleaved homolytically, putting a radical unpaired electron on both species:
(1) homocysteine–S:N–O
(2) homocysteine–S· + ·N–O
(2) homocysteine–S· + ·N–O
This radical electron (·) can shift to the α-carbon, an event catalyzed by sodium and potassium. This forms the stable free radical which can cross the blood–brain barrier, being nearly indistinguishable from normal homocysteine. Like a Trojan Horse it gains access to the highly-unsaturated brain lipids causing a lipid peroxidation cascade, ostensibly why you have a Pearson coefficient of .92 between cerebospinal homocysteine and hydroxynonenal in same fluid. Hydroxynonenal is a lipid peroxidation product capable of crosslinking proteins.
So I view homocysteine as a molecule capable of transporting radicals formed in the periphery into the brain. But for this to occur, the initial sulfur radical must form through an interaction with nitric oxide—another reason to avoid nitrates.
And like cyclooxygenase and phospholipase A₂, the enzyme nitric oxide synthase is also inducible by cytokines (iNOS). This enzyme cleaves nitric oxide off of arginine forming citrulline, and eventually ornithine upon further reactions.
Although it's common to measure plasma homocysteine, it is unheard of to determine the plasma concentration of the radical subspecies. This would be interesting to look into. The radical species is generally given credit for homocysteine dementia, although homocysteine can also form a cyclic thiolactone as well as modifying proteins directly (homocysteinylation).
Osburn, Sandra. "Structure and reactivity of homocysteine radical cation in the gas phase studied by ion–molecule reactions and infrared multiple photon dissociation." The Journal of Physical Chemistry (2012)
Lesslie, Michael "Alkali‐Metal‐Ion‐Assisted Hydrogen Atom Transfer in the Homocysteine Radical." Chemistry-A European Journal (2016)
Selley, M. L. "The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer’s disease." Neurobiology of aging (2002)
Lesslie, Michael "Alkali‐Metal‐Ion‐Assisted Hydrogen Atom Transfer in the Homocysteine Radical." Chemistry-A European Journal (2016)
Selley, M. L. "The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer’s disease." Neurobiology of aging (2002)
Last edited:
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
This is true. We did look into this on another thread. I think it had been estimated that over 50% of endogenous SAM‐derived methyl groups go to biosynthesize creatine. Some people see creatine more as a molecule which 'spares' methyl groups than a molecule which 'donates' them.Yes but it’s not necessarily mostly B12, B6, and folate (in your case it probably was) because you can also completely bypass the folate/b12 homocysteine recycling path and use only the BHMT path which is mostly about choline which converts to betaine. You can also probably significantly drop homocysteine without either the b vitamin path or BHMT paths by adding lots of creatine to your diet.
It's interesting to hear about that cobalamin‐independent pathway for regenerating methionine, using betaine.. . .
Last edited:
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
Another interesting find.Reminded me of this
J Physiol. 2017 Dec 19. doi: 10.1113/JP275075. [Epub ahead of print]
Restoration of metabolic health by decreased consumption of branched-chain amino acids.
Cummings NE1,2,3, Williams EM1,2, Kasza I4, Konon EN1,2, Schaid MD1,2,5, Schmidt BA1,2, Poudel C1,2, Sherman DS1,2, Yu D1,2,6, Arriola Apelo SI1,2,7, Cottrell SE1,2,8, Geiger G1,2, Barnes ME1,2, Wisinski JA1,2, Fenske RJ1,2,5, Matkowskyj KA2,9,10, Kimple ME1,2,3,5,11, Alexander CM4, Merrins MJ1,2,12, Lamming DW1,2,3,5,6,9.
KEY POINTS:
We recently found that feeding healthy mice a diet with reduced levels of branched-chain amino acids (BCAAs), which are associated with insulin resistance in both humans and rodents, modestly improves glucose tolerance and slows fat mass gain. In the present study, we show that a reduced BCAA diet promotes rapid fat mass loss without calorie restriction in obese mice. Selective reduction of dietary BCAAs also restores glucose tolerance and insulin sensitivity to obese mice, even as they continue to consume a high-fat, high-sugar diet. A low BCAA diet transiently induces FGF21 (fibroblast growth factor 21) and increases energy expenditure. We suggest that dietary protein quality (i.e. the precise macronutrient composition of dietary protein) may impact the effectiveness of weight loss diets.
ABSTRACT:
Obesity and diabetes are increasing problems around the world, and although even moderate weight loss can improve metabolic health, reduced calorie diets are notoriously difficult to sustain. Branched-chain amino acids (BCAAs; leucine, isoleucine and valine) are elevated in the blood of obese, insulin-resistant humans and rodents. We recently demonstrated that specifically reducing dietary levels of BCAAs has beneficial effects on the metabolic health of young, growing mice, improving glucose tolerance and modestly slowing fat mass gain. In the present study, we examine the hypothesis that reducing dietary BCAAs will promote weight loss, reduce adiposity, and improve blood glucose control in diet-induced obese mice with pre-existing metabolic syndrome. We find that specifically reducing dietary BCAAs rapidly reverses diet-induced obesity and improves glucoregulatory control in diet-induced obese mice. Most dramatically, mice eating an otherwise unhealthy high-calorie, high-sugar Western diet with reduced levels of BCAAs lost weight and fat mass rapidly until regaining a normal weight. Importantly, this normalization of weight was mediated not by caloric restriction or increased activity, but by increased energy expenditure, and was accompanied by a transient induction of the energy balance regulating hormone FGF21 (fibroblast growth factor 21). Consumption of a Western diet reduced in BCAAs was also accompanied by a dramatic improvement in glucose tolerance and insulin resistance. Our results link dietary BCAAs with the regulation of metabolic health and energy balance in obese animals, and suggest that specifically reducing dietary BCAAs may represent a highly translatable option for the treatment of obesity and insulin resistance.
I think amino acid ratios are more important than people give them credit for. Tryptophan, histamine, and tyrosine all cross the blood–brain barrier and form their respective neurotransmitters: serotonin, histamine, and dopamine. Now we have leucine to consider, being capable of inducing insulin and cholesterol synthesis.
Excess methionine greatly shortens the lifespans of rats by ~44% and causes weight gain, ostensibly through forming polyamines (I don't think homocysteine can explain the weight gain). Arginine is the nitric oxide precursor, and excess threonine can become methylgloxal (which can adduct with arginine.)
Last edited:
noordinary
Member
- Joined
- Jun 1, 2016
- Messages
- 209
Yes yes yes, more on this please! would be very informative and interestingExcess methionine greatly shortens the lifespans of rats by ~44% and causes weight gain, ostensibly through forming polyamines (I don't think homocysteine can explain the weight gain). Arginine is the nitric oxide precursor, and excess threonine can become methylgloxal (which can adduct with arginine.)
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
There's been at least three old studies on this. One of them, and I think it was Richie's, had a lifespan reduction of 44% with just a small increase in methionine. I believe this was replicated shortly afterwords by Orentriech, and then again shortly later by someone else. The reductions in lifespan noted in the follow‐up studies were less dramatic, yet still shocking (20–35%). These rats had also gained weight; in fact, you could no longer see their little legs: All you could see were a few claws projecting out of these soft and nearly spherical balls of hair formerly known as the 'test rats.' (lol, kidding!)Yes yes yes, more on this please! would be very informative and interesting
Here are a few excerpts, from Richie:
'To test this, blood and tissue GSH levels were measured at different ages throughout the life span in F344 rats on control or Met-restricted diets. Met restriction resulted in a 42% increase in mean and 44% in- crease in maximum life span, and in 43% lower body weight compared to controls.' ―Richie
'A recent report (26) described another dietary intervention that enhanced life span in mammals: increases in both median and maximum life span were observed in F344 rats fed a diet severely...' ―Richie
Woops! It looks like Orentriech did it first, published one year before Richie's. Now a few quotes from Orentreich:
'We report here that lifelong reduction in the concentration of a single dietary component, the essential amino acid L-methionine, from 0.86 to 0.17% of the diet results in a 30% longer life span of male Fischer 344 rats.' ―Orentriech
'Effect of dietary methionine on growth. Rats fed 0.86% methionine from 42 d of age gained nearly 350 g during the next 50 wk of the experiment. On the other hand, rats fed a 0.17% methionine diet from 42 d of age failed to gain weight throughout their lives (Fig. 2).' ―Orentriech
Despite eating nearly exactly the same amount of food, Orentreich's rats were 4× heavier:
But no mention of 'polyamines' in either article, the small molecule growth factor which converts DNA in the Z‐configuration and increases the replication rate of DNA during routine PCR. Polyamines also bind to microtubules, which need to be disassembled before mitosis.
Although polyamines can be made from lysine and arginine, the enzyme ornithine decarboxylse has the greatest affinity for methionine; so much so in fact, that polyamine synthesis from lysine and arginine are considered minor pathways. Lysine, for instance, has been shown in vitro to have a much lower affinity for ornithine decarboxylase.
The explanations given by Richie and Orentrieich centre around glutathione. Even though this is necessary for cell division, and can be considered a 'growth factor' in a way, glutathione cannot explain why cysteine doesn't reduce the lifespan and cause weight gain. The amino acid cysteine is an even more direct precursor for glutathione.
Some have even tried to explain this phenomenon through DNA methylation, which is even less convincing since choline will not do this (and saying nothing of the weak correlations found in all DNA methylation studies).
And, of course, 'oxidative stress' is sometimes invoked. This is a lazy explanation always used in an off‐hand way to explain everything, and everything, but doesn't seem to be able to explain much besides lipid peroxidation. While true that the spontaneous oxidation products of fatty acids can be toxic and hormonal, it takes cyclooxygenase to make prostaglandins. If they had checked for hydroxynonenal in the rates—or lipofuscin upon autopsy—I think they would have ruled this out, to a degree. While true that more methionine could lead to more homocysteine, this only catalylzes lipid peroxidation when demethylated—and only after homolytic cleavage of its nitric oxide adduct followed by intramolecular hydride shift.
I think it was the polyamines that inflated these rats into balls of hair, or sphericized them, completely unrecognizable were it not for that tail snaking out—wiggling with delight everytime it overhears Orentriech talking about lunch.
Richie, John P. "Methionine restriction increases blood glutathione and longevity in F344 rats." The FASEB Journal (1994)
Orentreich, Norman. "Low methionine ingestion by rats extends life span." The Journal of nutrition (1993)
Orentreich, Norman. "Low methionine ingestion by rats extends life span." The Journal of nutrition (1993)
Last edited:
Koveras
Member
- Joined
- Dec 17, 2015
- Messages
- 720
What are your thoughts on acrylamide?
Terma
Member
- Joined
- May 8, 2017
- Messages
- 1,063
(sorry if I didn't reply to older posts, it's in the past now)
The methionine restriction also jumped out at me about lowering fat mass, but I had no idea about which mechanism is biggest contributor to this.
This is great, Dec 2017, thank you. Alternative hypothesis for issues caused by leucine (& others): mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis
Do you mean as a substrate? Although I know MET is factor in polyamine synthesis (SAM decarboxylase + other), I've never heard anyone say it was that close to ODC. But we don't read all the same articles and I never got deep into polyamines.Although polyamines can be made from lysine and arginine, the enzyme ornithine decarboxylse has the greatest affinity for methionine; so much so in fact, that polyamine synthesis from lysine and arginine are considered minor pathways. Lysine, for instance, has been shown in vitro to have a much lower affinity for ornithine decarboxylase.
The methionine restriction also jumped out at me about lowering fat mass, but I had no idea about which mechanism is biggest contributor to this.
Reminded me of this
J Physiol. 2017 Dec 19. doi: 10.1113/JP275075. [Epub ahead of print]
Restoration of metabolic health by decreased consumption of branched-chain amino acids.
Cummings NE1,2,3, Williams EM1,2, Kasza I4, Konon EN1,2, Schaid MD1,2,5, Schmidt BA1,2, Poudel C1,2, Sherman DS1,2, Yu D1,2,6, Arriola Apelo SI1,2,7, Cottrell SE1,2,8, Geiger G1,2, Barnes ME1,2, Wisinski JA1,2, Fenske RJ1,2,5, Matkowskyj KA2,9,10, Kimple ME1,2,3,5,11, Alexander CM4, Merrins MJ1,2,12, Lamming DW1,2,3,5,6,9.
KEY POINTS:
We recently found that feeding healthy mice a diet with reduced levels of branched-chain amino acids (BCAAs), which are associated with insulin resistance in both humans and rodents, modestly improves glucose tolerance and slows fat mass gain. In the present study, we show that a reduced BCAA diet promotes rapid fat mass loss without calorie restriction in obese mice. Selective reduction of dietary BCAAs also restores glucose tolerance and insulin sensitivity to obese mice, even as they continue to consume a high-fat, high-sugar diet. A low BCAA diet transiently induces FGF21 (fibroblast growth factor 21) and increases energy expenditure. We suggest that dietary protein quality (i.e. the precise macronutrient composition of dietary protein) may impact the effectiveness of weight loss diets.
ABSTRACT:
Obesity and diabetes are increasing problems around the world, and although even moderate weight loss can improve metabolic health, reduced calorie diets are notoriously difficult to sustain. Branched-chain amino acids (BCAAs; leucine, isoleucine and valine) are elevated in the blood of obese, insulin-resistant humans and rodents. We recently demonstrated that specifically reducing dietary levels of BCAAs has beneficial effects on the metabolic health of young, growing mice, improving glucose tolerance and modestly slowing fat mass gain. In the present study, we examine the hypothesis that reducing dietary BCAAs will promote weight loss, reduce adiposity, and improve blood glucose control in diet-induced obese mice with pre-existing metabolic syndrome. We find that specifically reducing dietary BCAAs rapidly reverses diet-induced obesity and improves glucoregulatory control in diet-induced obese mice. Most dramatically, mice eating an otherwise unhealthy high-calorie, high-sugar Western diet with reduced levels of BCAAs lost weight and fat mass rapidly until regaining a normal weight. Importantly, this normalization of weight was mediated not by caloric restriction or increased activity, but by increased energy expenditure, and was accompanied by a transient induction of the energy balance regulating hormone FGF21 (fibroblast growth factor 21). Consumption of a Western diet reduced in BCAAs was also accompanied by a dramatic improvement in glucose tolerance and insulin resistance. Our results link dietary BCAAs with the regulation of metabolic health and energy balance in obese animals, and suggest that specifically reducing dietary BCAAs may represent a highly translatable option for the treatment of obesity and insulin resistance.
This is great, Dec 2017, thank you. Alternative hypothesis for issues caused by leucine (& others): mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
You got me. I was thinking about ornithine being a better substrate than lysine, not methionine:(sorry if I didn't reply to older posts, it's in the past now)
Do you mean as a substrate? Although I know MET is factor in polyamine synthesis (SAM decarboxylase + other), I've never heard anyone say it was that close to ODC. But we don't read all the same articles and I never got deep into polyamines.
The methionine restriction also jumped out at me about lowering fat mass, but I had no idea about which mechanism is biggest contributor to this.
This is great, Dec 2017, thank you. Alternative hypothesis for issues caused by leucine (& others): mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis
'A highly purified ornithine decarboxylase preparation was able to decarboxylate lysine and the ratio of ornithine to lysine decarboxylase activities was constant throughout purification. Kinetic studies of the purified preparation showed that the V for ornithine was about 4-fold greater than for lysine...' ―Pegg
So lysine can make a diamine through ornithine decarboxylase, called cadaverine, but this appears to be a red herring (even though cadaverine is consistently classified as a 'polyamine' on many tables and charts.) Cadaverine isn't a good substrate for spermidine synthase, although an unconventional polyamine can be made from 1,4‐diamino‐2‐butene (unsaturated putrescine).
 click to embiggen: Image showing decarboxylated lysine diamine (cadaverine) unsuitable for polyamine synthesis.
click to embiggen: Image showing decarboxylated lysine diamine (cadaverine) unsuitable for polyamine synthesis.So we have the initial putrescine, onto which are added two aminoproply groups—one to each end—donated by S‐adenosylmethionine (forming spermine). For the most active polyamine to be synthesized, a cell needs two methionines for every one ornithine.
Spermidine is made from just one decarboxylated methionine (aminopropyl–) and one decarboxylated ornithine (putrescine)—chained together—but this is not as effective as the longer spermine in catalyzing DNA synthesis, nor in binding microtubules. The data on interactions with DNA far outnumber the few studies on polyamines and microtubules so it could be hard to know what to make of this, by you might think this could have relevance since microtubules need to be disassembled for mitosis to occur.
The calcium ion destabilizes microtubules and inhibits the polymerization of purified tubulin in vitro, necessitating the use of EDTA for efficient microtubule growth. Perhaps an intracellular calcium spike could be what initiates mitosis?—the Ca²⁺ ions perhaps percolating towards the microtubules and interposing themselves between 'salt bridges,' disrupting the carboxyl–amine ionic attractions. It's well known that proteins can be denatured by ions—at least to Gilbert Ling—as their intramolecular hydrogen bonds and 'salt bridges' (–N₃⁺ ⁻O–) are disrupted. The disulfide bridge can be broken by electrons as the thiols are reduced, a fact as true in proteins as in glutathione disulfide (oxidized glutathione). The popular transcription factor p53 has an intramolecular disulfide domain (when oxidized) which is thought to constitute a redox‐active switch by some—a sensor for oxidative stress. In this way p53 could be released from its binding protein only in the event of electron imbalance, reactive nitrogen species, or hydrogen peroxide—initiating its well‐characterized apoptosis cascade in that manner:
'The OxyR protein contains a thiol–disulfide redox switch to sense hydrogen peroxide. The SoxR protein uses a 2Fe–2S cluster to sense superoxide generated by redox-cycling agents, as well as to sense nitric oxide.' ―Zheng
Not surprisingly, redox‐sensitive transcription factors in the nucleus transcribe for mRNA which act to maintain homestasis:
'The E. coli OxyR transcription factor activates the expression of several antioxidant defensive genes in response to elevated levels of hydrogen peroxide [2, 3], including katG (hydroperoxidase), ahpCF (alkyl hydroperoxide reductase), oxyS (a regulatory RNA), dps (a non-specific DNA-binding protein), fur (ferric uptake regulation), gorA (glutathione reductase), and grxA (glutaredoxin).' ―Zheng
And this has been, more‐or‐less, experimentally proven:
'Early studies demonstrated that OxyR from E. coli exists in oxidized and reduced forms, but that only the former activates transcription [5]. Recent studies showed that oxidation by peroxide leads to the formation of a disulfide bond in OxyR that triggers the activation of the transcription factor [6]. Mutation of either of the two conserved cysteines (C199 and C208) in OxyR to serine or alanine abolished the ability of the transcription factor to sense hydrogen peroxide, both in vivo and in vitro. Mass spectrometry measurements and thiol–disulfide quantitation assays on the purified protein showed that C199 and C208 are in disulfide bond form in the oxidized OxyR, and in dithiol form in the reduced OxyR. These findings suggest that OxyR activation and deactivation is a consequence of the C199–C208 disulfide bond formation and reduction (Scheme 1).' ―Zheng
And with a nod to p53 at the end.. . .
'The dual roles of sensing divalent metal ions and sensing peroxides exhibited by the PerR protein is reminiscent of similar properties shown by the eukaryotic p53 [26] and MTF1 [27] transcription factors.' ―Zheng
And Thronally has provided evidence of a transcription factor being under the control of methylglyoxal through an arginine adduct (the imidazalone cycloaddition, also responsible for corneal refractive changes and cataracts).
Transcription factors are pretty amazing. Besides through the binding of steroids, lipids, and prostaglandins, transcription factors can be activated by electrons (i.e. p53) and by methylglyoxal (through corepressor Sin3)—sensing glucose metabolism in this manner. High levels of methylgloxal could be expected to decrease both nitric oxide and polyamine de novo biosynthesis by lowering the free arginine ratio. And also, many glycolytic enzymes—such as GAPDH and pyruvate decarboxylase—have an arginine in their catalytic domain which binds the pyrophosphate domain of their cofactor (NAD(P)H). This means any NADH‐dependent enzyme lacking cofactor could perhaps be incapacitated by methylglyoxal, a negative feedback mechanism controlling the rate of glycolysis.
So to know how microtubules are disassembled before mitosis, perhaps all that is needed to know is how tubulin monomers are connected?
Zheng, Ming. "Redox sensing by prokaryotic transcription factors." Biochemical pharmacology (2000)
Ling, Gilbert. "A Physical Theory of the Living State: The Association-Induction Hypothesis." Blaisdell (1963)
Pegg, Anthony E. "Decarboxylation of ornithine and lysine in rat tissues." Biochimica et Biophysica Acta (BBA)-Enzymology (1979)
Tabor, Herbert. "The biosynthesis of spermidine and spermine from putrescine and methionine." Journal of Biological Chemistry (1958)
Thornally, Paul. "High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A." Journal of Biological Chemistry (2007)
Ling, Gilbert. "A Physical Theory of the Living State: The Association-Induction Hypothesis." Blaisdell (1963)
Pegg, Anthony E. "Decarboxylation of ornithine and lysine in rat tissues." Biochimica et Biophysica Acta (BBA)-Enzymology (1979)
Tabor, Herbert. "The biosynthesis of spermidine and spermine from putrescine and methionine." Journal of Biological Chemistry (1958)
Thornally, Paul. "High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A." Journal of Biological Chemistry (2007)
Last edited:
EMF Mitigation - Flush Niacin - Big 5 Minerals
Similar threads
- Replies
- 38
- Views
- 5K
- Replies
- 2
- Views
- 3K
- Replies
- 27
- Views
- 4K
- Replies
- 30
- Views
- 4K
- Replies
- 29
- Views
- 10K
- Replies
- 40
- Views
- 9K
- Replies
- 11
- Views
- 5K
- Replies
- 5
- Views
- 2K
- Replies
- 1
- Views
- 2K
- Replies
- 23
- Views
- 2K
- Replies
- 7
- Views
- 854
- Replies
- 142
- Views
- 16K