Amazoniac
Member
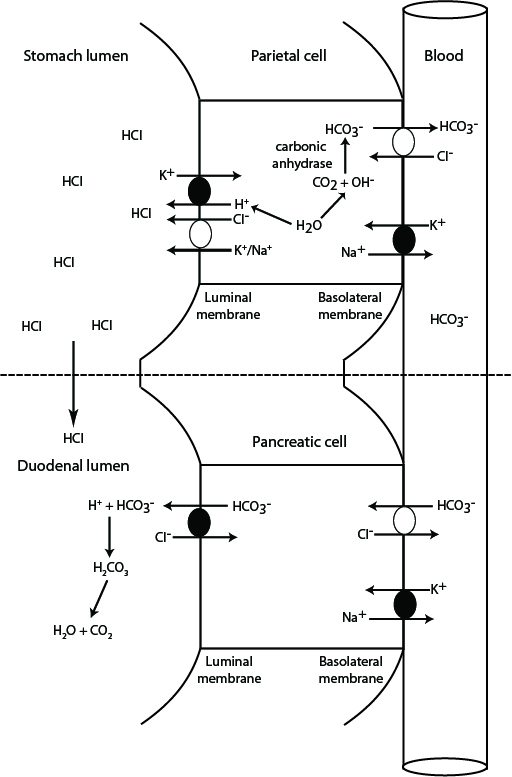
Possibly (for now I'll have to take their word for it, I don't understand how agmatine works for this purpose yet).Can't the starting source be the usual suspect - not from pepsinogen, but from vascular carbonic acid? That would act like the spark that initiates a chain reaction in the gut walls where the activation segment is activated , and this perpetuates the acidity needed to enable a chain reaction of producing carbon dioxide and agmatine, and the agmatine would provide the alkaline environment for carbon dioxide to turn into carbonic acid for use as substrate for making HCl?
Perhaps sourcing crapon dioxide this way contributes to increase the acidity when large amounts of protein are being digested. The 'patchy distribution' of pepsinogen would require something else in cells without it, explaining the acidity and protecting them against it.
Thanks for insisting on the topic, otherwise I would have dismissed it.