The skinny: The infamous Boulware study fraudulently used Folate as placebo to claim HCQ was ineffective, when they KNEW that folic acid/folate were as effective as HCQ!

 communityoperatingsystem.wordpress.com
communityoperatingsystem.wordpress.com
In the history of science and medicine it is amazing how many advances have taken place by accident. In terms of medicine: X-Rays, Penicillin, Insulin, Quinine and the pacemaker are just some stand out examples and I fully expect there to be a good deal more if people were more honest as to the precise history of how things came about. It is more often the case that a governing ‘intention’ is inserted after the accident in order to create an illusion of authority as if one were somehow fully in ‘power’ over one’s discoveries or indeed creativity and coming into being in general.
The problem with recognising and taking advantage of any accidental outcome is that one has to apply a specific methodology in how one deals with unexpected findings that might arise from research development and it is often the case that the scientists involved have a tendency to become too strictly governed by the parameters of their immediate brief and fail to notice or try and explain glaring anomalies that might be thrown up in the course of their experiments.
One such recent experimental medical study that I believe falls into this highly restrictive interpretation of its own results was the Boulware study “A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19“.
What was unusual about this study was that they chose Folate as the placebo given to the control group: supposedly on account of the fact that it was similar in appearance to the HCQ tablets. “Placebo folate tablets, which were similar in appearance to the hydroxychloroquine tablets, were prescribed as an identical regimen for the control group“. This choice of placebo was not mentioned in the study protocol which was published on the 17th March so we do not know when this placebo selection was made.
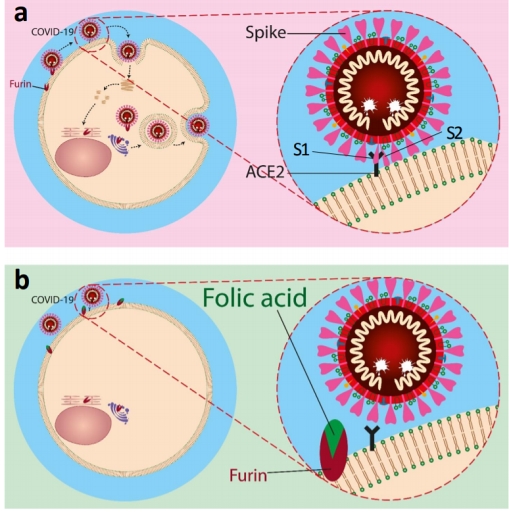
However, on the 30th March the paper The Role of Folic Acid in the Management of Respiratory Disease Caused by COVID-19 was published giving a highly detailed overview of how folic acid acts as a blocker or inhibitor in relation to the ACE2 furin enzyme and the furin cleavage site that makes Covid-19 so contagious and unique amongst coronavirus.
The study makes the following conclusion.
“In summary, our results suggest that folic acid could be used to inhibit the furin enzyme. The association of folic acid with furin would affect the structure of the protein and consequently interfere with its proteolytic capability. Thus, folic acid, as a safe drug, could be useful in the prevention or management of COVID-19-associated respiratory disease in the early stages of the disease.”
Folate as the biologically active form of folic acid was clearly a candidate for a treatment study in its own right. In terms of bio-chemistry part of the reason that HCQ and Quercetin have been seen as a useful treatment for Covid-19 is because they act as a gateway for positively charged Zinc ions to enter cells and Zinc has been shown to inhibit viral replication of Sars-CoV once the virus has entered a cell via the furin cleavage site. It seems logical then, that a cheap and completely safe prophylactic integrated protocol of Folate, Quercetin and Zinc Sulphate should therefore be a highly effective combined prophylactic for Covid-19 in that the folate initially blocks the virus from binding to the furin site and zinc would then act as a secondary line of defence in that it would inhibit the viral replication of any virus that did manage to bind successfully.
All in all then, there are very good reasons to assume that Folate is likely to be a highly effective prophylactic and treatment for Covid-19. In which case it would be interesting to evaluate the outcomes of the Boulware Postexposure Prophylaxis study with the assumption that folate could well have been an effective Covid-19 treatment even if its was ‘accidentally’ employed in the control arm of the study.
If all of the above is true then it raises important issues if we examine the hypothesis for the Boulware study in that it was designed to prove that HCQ was superior to Folate both in terms of its preventing progression to Covid-19 disease and in terms of preventing hospitalisation and mortality.
The image below is taken from a screen shot of the protocol title page. It clearly cites Preemptive Therapy as a dual concern.

The image below is taken from a screen shot of the title of the final published paper.
It is immediately obvious that the secondary aim of the study had been entirely excluded from the title of the final paper. There is no discussion or even mention of either the secondary investigation into preemptive therapy or ameliorating the severity of the disease. None of these terms appear in the final paper yet the terms preemptive and the precise term ‘preemptive therapy’ appears no less than 17 times in the protocol whilst ameliorating severity of the disease appears in various forms on 7 occasions.
In the final paper we find the study hypothesis has been reduced to just the single evaluation of prophylaxis.
https://www.nejm.org/doi/full/10.1056/NEJMoa2016638?query=featured_home
Boulware Study Protocol
In this case, the authors conservatively predicted that 10% of participants who contracted COVID-19 in the control group who were receiving Folate [placebo] would go on to develop a severe enough case of the disease as to require hospital treatment and of that 10%, 2% would require ICU treatment with a possibility of fatality.
In a massive miss in terms of their predicted outcomes we find that by the time the final paper was published, that of the 107 participants who deemed to have contracted COVID-19 that just “Two hospitalizations were reported (one in each group). No arrhythmias or deaths occurred“.
That would put the hospitalization rate for both arms of the study at 2% and not the predicted 10%. However, having downloaded the study data which has since been made available, I find that the Boulware was being entirely disingenuous in his reporting of hospitalizations
The female subject in the HCQ group who was admitted to hospital did not test positive with a PCR in hospital or from any follow up – therefore she could not have had Covid-19 but more likely a regular flu type illness.
The black male subject in the control group who was admitted to hospital did test PCR positive but he did not take the folate and is recorded as having missed taking all 19 pills.
This means that in terms of reducing the severity of Covid-19 by taking either folate or HCQ, both agents reduced hospitalization rates to 0% when the study protocol had conservatively predicted that 10% of participants who became symptomatic would require hospital admission, with 2% requiring admission to ICU. 107 subjects were judged to have Covid-19 so they should have seen at least 10 admissions given their conservative rate (the admission rate in the US at the time was running at 14%). Both HCQ and folate in each arm of the study eliminated the need for Covid-19 hospitalization entirely! This was an absolutely stunning result which by far outstripped any RCT treatment study for Covid-19 done either before or since.
There is a rather massive and glaring data anomaly between their 10% control group hospitalisation prediction and the actual zero % outcome which the authors artfully concealed from the public by sleight of hand. It should be noted here that this study was carried out between early 17th March and May 6th and that during this time records show that the US was at its peak in daily new cases and daily deaths.
What one cannot ignore is that during the height of the pandemic in the US, this study gave two different potential prophylactic treatments to the two different arms of its study and in both those arms the net result was the complete elimination of cases that required hospitalisation for Covid-19. In a study that originally claimed to be interested in evaluating whether “early preemptive therapy may ameliorate disease severity” it is deeply shocking that the authors chose not to fully evaluate their own findings and at least ask the properly scientific question as to WHY the actual outcomes deviated so greatly from their original predictions and expectations? It beggars belief that given such results they terminated the study early claiming it was futile simply because one preemptive treatment did not show itself to be statistically more beneficial than the other preemptive treatment or that one prophylaxis to not show itself to be superior to the other similarly effective prophylaxis.
This outcome was always highly questionable in that there was an unacceptable time lag between exposure and the receipt of treatments and even in vitro experiments cited in the protocol showed that far greater quantities of HCQ were required in vitro merely after a 5 hour delay to achieve a similar result to that of immediate application. Indeed the authors were well aware in advance that the efficacy of showing prophylaxis was intensely problematic given the extremely tight window or time frame involved. It was this factor itself that induced them into including two aims in the study. Prophylaxis AND preemptive therapy.
If HCQ and Folate had been put into two separate studies with control groups using genuine placebos in order to determine whether either had any beneficial therapeutic effects which ameliorated the severity of the disease: then the result would have been a resounding success in both trials. It was as inappropriate to have included folate as a placebo for such an RCT as it would have been to have included Remdesivir as a placebo. The trial compared apples with apples and then declared that the fruit was of no dietary benefit.
Given the continued global threat of Covid-19 then there is a pressing need to quickly evaluate the efficacy of people taking quercetin, zinc, vitamins C & D as supplements along with Folate in an integrated prophylactic for Covid-19. Patients who actually contract the disease could then be immediately given HCQ in substitution for Quercetin as HCQ is a more effective zinc ionophore.
If folic acid/Folate does provide any therapeutic benefits we would expect it to be most marked in the group in their first trimester of pregnancy and predict that its effect would wane over the second and third trimesters after women had stopped taking it and the levels of folic acid declined in their bodies. We would expect to find less symptomatic patients amongst the first trimester group and a lower incidence of serious illness and hospitalisations. Conversely we would expect to find a greater degree of illness severity for those in their second and third trimesters or for those who did not follow advice and failed to take folic acid in their first trimester.
With this hypothesis in mind I searched for reports on how pregnant women were faring against COVID-19 but found nothing definitive. Finally on June 19th, I found this report at the British Medical Journal by Professor Marian Knight which gave added weight to my assumptions and hypothesis about the possible therapeutic benefits of Folate/folic acid in that out of all pregnant women admitted to hospital: 81% of those were in the third trimester of their pregnancy. This statistical anomaly underlines the hypothesis that women were more likely to be progressively severely effected in their second and third trimesters as Folate levels declined in their bodies since they stopped taking it.
Unfortunately the study fails to report the precise percentage of those who were admitted in their first trimester and of those who may have been admitted we have no knowledge at this time as to whether they had followed medical advice and had taken folic acid or not. It would certainly be worth following up that small number of patients and asking whether they took folic acid or not. However, 19% of women admitted must have been in their second or first trimester and if most of the general cohort were in their late second and third trimesters then it is reasonable to speculate that those in their early second trimester would have been small and those in their first trimester would have been a very small percentage indeed.
I have read claims that pregnant women in their third trimester are more likely to admitted to hospital with influenza than other trimesters but this is only in some strains of influenza and the differences are marginal. This is confirmed by a very comprehensive study “Epidemiology of influenza in pregnant women hospitalized with respiratory illness in Moscow, 2012/2013–2015/2016: a hospital-based active surveillance study” which analysed PCR confirmed influenza admissions at a Moscow Clinical Hospital for Infectious Diseases which specialises in treating pregnant women.
The study was carried out analysing data from 4 influenza seasons. The average percentage of influenza admissions for 4 different flu strains for pregnant women in their third trimester was 35%. Pregnant women in their second trimester were the most at risk group with an average of 44% of all admissions. The highest admission rate of third trimester patients for an individual strain of influenza A(H3N2) was 43% in comparison which is well below the 81% recorded with Covid-19.
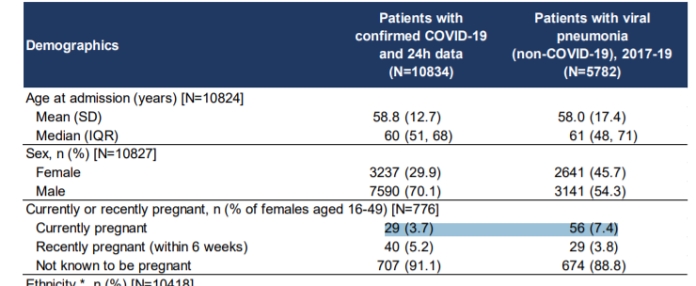
Fortunately, this does not mean that third trimester pregnant women are especially prone to Covid-19 and that it is more than likely that Folate carries on protecting them to a certain extent. The ICNARC COVID-19 report 2020-09-07.pdf: which compares the demographics of Covid-19 currently pregnant admissions to ICU in comparison with a cohort of currently pregnant patients admitted with viral pneumonia from 2017-19: clearly shows that of 776 female Covid-19 patients admitted to ICU between the ages of 16 and 49, then in comparative terms, the good news is that currently pregnant women are 50% less likely to be admitted to ICU with Covid-19 than they are with viral pneumonia or influenza like illnesses. Men on the other hand are 142% more likely to be admitted to ICU with Covid-19 than viral pneumonia.
It should also be noted that “233 (56%) pregnant women admitted to hospital with SARS-CoV-2 infection in pregnancy were from black or other ethnic minority groups, 281 (69%) were overweight or obese, 175 (41%) were aged 35 or over, and 145 (34%) had pre-existing comorbidities.”
Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study
In conclusion we have a bio-chemical explanation of how folic acid impedes Covid-19 from binding to ACE2 receptors as a first line of defence. We also have evidence in a randomised control trial of the general public who were given Folate as a placebo and hospitalizations fell to zero, below the study designers expectations. Now we also have data on how pregnant women in their first trimester seem to be virtually unaffected by COVID-19: all of which collectively suggest that both HCQ and Folate/Folic Acid are important, cheap and safe therapeutic agents which are worthy of deeper medical research and immediate addition to integrated Covid-19 treatment protocols.

HCQ and Folate/Folic Acid are effective prophylaxis and preemptive treatments for Covid-19
In the history of science and medicine it is amazing how many advances have taken place by accident. In terms of medicine: X-Rays, Penicillin, Insulin, Quinine and the pacemaker are just some stan…
In the history of science and medicine it is amazing how many advances have taken place by accident. In terms of medicine: X-Rays, Penicillin, Insulin, Quinine and the pacemaker are just some stand out examples and I fully expect there to be a good deal more if people were more honest as to the precise history of how things came about. It is more often the case that a governing ‘intention’ is inserted after the accident in order to create an illusion of authority as if one were somehow fully in ‘power’ over one’s discoveries or indeed creativity and coming into being in general.
The problem with recognising and taking advantage of any accidental outcome is that one has to apply a specific methodology in how one deals with unexpected findings that might arise from research development and it is often the case that the scientists involved have a tendency to become too strictly governed by the parameters of their immediate brief and fail to notice or try and explain glaring anomalies that might be thrown up in the course of their experiments.
One such recent experimental medical study that I believe falls into this highly restrictive interpretation of its own results was the Boulware study “A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19“.
What was unusual about this study was that they chose Folate as the placebo given to the control group: supposedly on account of the fact that it was similar in appearance to the HCQ tablets. “Placebo folate tablets, which were similar in appearance to the hydroxychloroquine tablets, were prescribed as an identical regimen for the control group“. This choice of placebo was not mentioned in the study protocol which was published on the 17th March so we do not know when this placebo selection was made.
However, on the 30th March the paper The Role of Folic Acid in the Management of Respiratory Disease Caused by COVID-19 was published giving a highly detailed overview of how folic acid acts as a blocker or inhibitor in relation to the ACE2 furin enzyme and the furin cleavage site that makes Covid-19 so contagious and unique amongst coronavirus.

The study makes the following conclusion.
“In summary, our results suggest that folic acid could be used to inhibit the furin enzyme. The association of folic acid with furin would affect the structure of the protein and consequently interfere with its proteolytic capability. Thus, folic acid, as a safe drug, could be useful in the prevention or management of COVID-19-associated respiratory disease in the early stages of the disease.”
Folate as the biologically active form of folic acid was clearly a candidate for a treatment study in its own right. In terms of bio-chemistry part of the reason that HCQ and Quercetin have been seen as a useful treatment for Covid-19 is because they act as a gateway for positively charged Zinc ions to enter cells and Zinc has been shown to inhibit viral replication of Sars-CoV once the virus has entered a cell via the furin cleavage site. It seems logical then, that a cheap and completely safe prophylactic integrated protocol of Folate, Quercetin and Zinc Sulphate should therefore be a highly effective combined prophylactic for Covid-19 in that the folate initially blocks the virus from binding to the furin site and zinc would then act as a secondary line of defence in that it would inhibit the viral replication of any virus that did manage to bind successfully.
All in all then, there are very good reasons to assume that Folate is likely to be a highly effective prophylactic and treatment for Covid-19. In which case it would be interesting to evaluate the outcomes of the Boulware Postexposure Prophylaxis study with the assumption that folate could well have been an effective Covid-19 treatment even if its was ‘accidentally’ employed in the control arm of the study.
If all of the above is true then it raises important issues if we examine the hypothesis for the Boulware study in that it was designed to prove that HCQ was superior to Folate both in terms of its preventing progression to Covid-19 disease and in terms of preventing hospitalisation and mortality.
It is worthwhile noting at this point, that in the study protocol, whilst the authors declared one of the aims of the study was to assess the possibility “for preventing progression among those with symptomatic mild COVID19 disease preventing hospitalization/death” and that HCQ might “ameliorate the severity of the disease”, it transpired that this secondary aim of their original hypothesis and protocol was entirely excluded from their final considerations and conclusions as we see in the final published study as their original hypothesis has been reduced to only evaluating the possibility of HCQ preventing symptomatic infection and we find that the possible outcome of HCQ preventing hospitalization and ameliorating the severity of the disease was entirely eliminated from their final report.11.1 Study Hypotheses
Hydroxychloroquine is superior to Folate [placebo] for preventing progression to COVID19 disease.
Hydroxychloroquine is superior to Folate [placebo] for preventing progression among those with symptomatic mild COVID19 disease preventing hospitalization/death.
The image below is taken from a screen shot of the protocol title page. It clearly cites Preemptive Therapy as a dual concern.

The image below is taken from a screen shot of the title of the final published paper.
It is immediately obvious that the secondary aim of the study had been entirely excluded from the title of the final paper. There is no discussion or even mention of either the secondary investigation into preemptive therapy or ameliorating the severity of the disease. None of these terms appear in the final paper yet the terms preemptive and the precise term ‘preemptive therapy’ appears no less than 17 times in the protocol whilst ameliorating severity of the disease appears in various forms on 7 occasions.
In the final paper we find the study hypothesis has been reduced to just the single evaluation of prophylaxis.
As I will show later, this is highly relevant to their later extrapolations as to which results of the study were taken to be significant and which results would be entirely ignored and assumed to be insignificant.We hypothesised that hydroxychloroquine could potentially be used as post-exposure prophylaxis, to prevent symptomatic infection after exposure to Covid-19.
https://www.nejm.org/doi/full/10.1056/NEJMoa2016638?query=featured_home
Boulware Study Protocol
Study Method
We conducted a randomized, double-blind, folate [placebo]-controlled trial across the United States and parts of Canada testing hydroxychloroquine as postexposure prophylaxis. We enrolled adults who had household or occupational exposure to someone with confirmed Covid-19 at a distance of less than 6 ft for more than 10 minutes while wearing neither a face mask nor an eye shield (high-risk exposure) or while wearing a face mask but no eye shield (moderate-risk exposure). Within 4 days after exposure, we randomly assigned participants to receive either Folate[placebo] or hydroxychloroquine (800 mg once, followed by 600 mg in 6 to 8 hours, then 600 mg daily for 4 additional days). The primary outcome was the incidence of either laboratory-confirmed Covid-19 or illness compatible with Covid-19 within 14 days.
The Protocol’s Key Assumptions and Estimates Concerning Outcomes For Symptomatic Patients
In order to judge a scientific theory or hypothesis we need to evaluate the accuracy of its predictions with the observable or documented results of the experiment. In the best science these will closely match each other as these are essential parameters in evaluating whether something worked or not.• 10% transmission rate from COVID-19 cases to close contacts
• For those with symptomatic illness the proportions at day 14 in the Folate [placebo] group are 90%, 8% and 2%, respectively for illness without hospitalization, hospitalization without ICU stay or death, and hospitalization with an ICU stay or death.
In this case, the authors conservatively predicted that 10% of participants who contracted COVID-19 in the control group who were receiving Folate [placebo] would go on to develop a severe enough case of the disease as to require hospital treatment and of that 10%, 2% would require ICU treatment with a possibility of fatality.
In a massive miss in terms of their predicted outcomes we find that by the time the final paper was published, that of the 107 participants who deemed to have contracted COVID-19 that just “Two hospitalizations were reported (one in each group). No arrhythmias or deaths occurred“.
That would put the hospitalization rate for both arms of the study at 2% and not the predicted 10%. However, having downloaded the study data which has since been made available, I find that the Boulware was being entirely disingenuous in his reporting of hospitalizations
The female subject in the HCQ group who was admitted to hospital did not test positive with a PCR in hospital or from any follow up – therefore she could not have had Covid-19 but more likely a regular flu type illness.
The black male subject in the control group who was admitted to hospital did test PCR positive but he did not take the folate and is recorded as having missed taking all 19 pills.
This means that in terms of reducing the severity of Covid-19 by taking either folate or HCQ, both agents reduced hospitalization rates to 0% when the study protocol had conservatively predicted that 10% of participants who became symptomatic would require hospital admission, with 2% requiring admission to ICU. 107 subjects were judged to have Covid-19 so they should have seen at least 10 admissions given their conservative rate (the admission rate in the US at the time was running at 14%). Both HCQ and folate in each arm of the study eliminated the need for Covid-19 hospitalization entirely! This was an absolutely stunning result which by far outstripped any RCT treatment study for Covid-19 done either before or since.
There is a rather massive and glaring data anomaly between their 10% control group hospitalisation prediction and the actual zero % outcome which the authors artfully concealed from the public by sleight of hand. It should be noted here that this study was carried out between early 17th March and May 6th and that during this time records show that the US was at its peak in daily new cases and daily deaths.
What one cannot ignore is that during the height of the pandemic in the US, this study gave two different potential prophylactic treatments to the two different arms of its study and in both those arms the net result was the complete elimination of cases that required hospitalisation for Covid-19. In a study that originally claimed to be interested in evaluating whether “early preemptive therapy may ameliorate disease severity” it is deeply shocking that the authors chose not to fully evaluate their own findings and at least ask the properly scientific question as to WHY the actual outcomes deviated so greatly from their original predictions and expectations? It beggars belief that given such results they terminated the study early claiming it was futile simply because one preemptive treatment did not show itself to be statistically more beneficial than the other preemptive treatment or that one prophylaxis to not show itself to be superior to the other similarly effective prophylaxis.
This outcome was always highly questionable in that there was an unacceptable time lag between exposure and the receipt of treatments and even in vitro experiments cited in the protocol showed that far greater quantities of HCQ were required in vitro merely after a 5 hour delay to achieve a similar result to that of immediate application. Indeed the authors were well aware in advance that the efficacy of showing prophylaxis was intensely problematic given the extremely tight window or time frame involved. It was this factor itself that induced them into including two aims in the study. Prophylaxis AND preemptive therapy.
As the incubation period is 2-14 days with a mean incubation period of 5-6 days, we seek to deliver post-exposure prophylaxis by the morning of day 4 at the latest. (<=3 days is an inclusion criteria). We recognize this may turn “post-exposure prophylaxis” into more of a “preemptive therapy” for some subjects who rapidly develop disease after trial randomization. If hydroxychloroquine does not prevent disease for some, preemptive therapy may ameliorate the COVID-19 disease severity.
Yet another HCQ study which was designed to fail
In many respects this study was doomed to fail by virtue of its hypothesis which demanded that HCQ was contractually obliged to show itself to be superior to Folate: whereas it turned out that both HCQ and Folate were equally shown to be highly effective in reducing the severity of the disease likely because folic acid inhibits the virus from ACE2 binding in a primary capacity and HCQ inhibits viral replication in a secondary capacity. Each treatment should be expected to reduce severity on its own terms and a combination of both would be better still. In evaluating two different medications with both having potential prophylactic and therapeutic benefits: the study effectively cancelled out each agent against the other and in omitting any discussion of the real zero rate of hospitalisations: they effectively suppressed the therapeutic benefits that their own randomised control trial had empirically demonstrated. The whole world could have benefited from two highly effective treatments for Covid-19 and thanks to the Boulware study it received neither. This deception has probably cost hundreds of thousands of lives globally.If HCQ and Folate had been put into two separate studies with control groups using genuine placebos in order to determine whether either had any beneficial therapeutic effects which ameliorated the severity of the disease: then the result would have been a resounding success in both trials. It was as inappropriate to have included folate as a placebo for such an RCT as it would have been to have included Remdesivir as a placebo. The trial compared apples with apples and then declared that the fruit was of no dietary benefit.
Given the continued global threat of Covid-19 then there is a pressing need to quickly evaluate the efficacy of people taking quercetin, zinc, vitamins C & D as supplements along with Folate in an integrated prophylactic for Covid-19. Patients who actually contract the disease could then be immediately given HCQ in substitution for Quercetin as HCQ is a more effective zinc ionophore.
An analysis of Covid-19, Folic Acid and Pregnancy
Fortunately we already have a very large group who have been taking folic acid as a medical protocol during the first 12 weeks of pregnancy. In the UK alone, we already have the richest data-set amongst many thousands of closely monitored and documented participants at any given time who happen to be pregnant, roughly 33% of whom will have been in the first trimester of pregnancy and the majority of that group should have been taking folic acid daily as advised.If folic acid/Folate does provide any therapeutic benefits we would expect it to be most marked in the group in their first trimester of pregnancy and predict that its effect would wane over the second and third trimesters after women had stopped taking it and the levels of folic acid declined in their bodies. We would expect to find less symptomatic patients amongst the first trimester group and a lower incidence of serious illness and hospitalisations. Conversely we would expect to find a greater degree of illness severity for those in their second and third trimesters or for those who did not follow advice and failed to take folic acid in their first trimester.
With this hypothesis in mind I searched for reports on how pregnant women were faring against COVID-19 but found nothing definitive. Finally on June 19th, I found this report at the British Medical Journal by Professor Marian Knight which gave added weight to my assumptions and hypothesis about the possible therapeutic benefits of Folate/folic acid in that out of all pregnant women admitted to hospital: 81% of those were in the third trimester of their pregnancy. This statistical anomaly underlines the hypothesis that women were more likely to be progressively severely effected in their second and third trimesters as Folate levels declined in their bodies since they stopped taking it.
Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort studyConclusions Most pregnant women admitted to hospital with SARS-CoV-2 infection were in the late second or third trimester…….Women had symptoms at a median of 34 (interquartile range 29-38) completed weeks’ gestation, with most women admitted to hospital having symptoms in the third trimester of pregnancy or peripartum (342/424; 81%)
Unfortunately the study fails to report the precise percentage of those who were admitted in their first trimester and of those who may have been admitted we have no knowledge at this time as to whether they had followed medical advice and had taken folic acid or not. It would certainly be worth following up that small number of patients and asking whether they took folic acid or not. However, 19% of women admitted must have been in their second or first trimester and if most of the general cohort were in their late second and third trimesters then it is reasonable to speculate that those in their early second trimester would have been small and those in their first trimester would have been a very small percentage indeed.
I have read claims that pregnant women in their third trimester are more likely to admitted to hospital with influenza than other trimesters but this is only in some strains of influenza and the differences are marginal. This is confirmed by a very comprehensive study “Epidemiology of influenza in pregnant women hospitalized with respiratory illness in Moscow, 2012/2013–2015/2016: a hospital-based active surveillance study” which analysed PCR confirmed influenza admissions at a Moscow Clinical Hospital for Infectious Diseases which specialises in treating pregnant women.
The study was carried out analysing data from 4 influenza seasons. The average percentage of influenza admissions for 4 different flu strains for pregnant women in their third trimester was 35%. Pregnant women in their second trimester were the most at risk group with an average of 44% of all admissions. The highest admission rate of third trimester patients for an individual strain of influenza A(H3N2) was 43% in comparison which is well below the 81% recorded with Covid-19.
Fortunately, this does not mean that third trimester pregnant women are especially prone to Covid-19 and that it is more than likely that Folate carries on protecting them to a certain extent. The ICNARC COVID-19 report 2020-09-07.pdf: which compares the demographics of Covid-19 currently pregnant admissions to ICU in comparison with a cohort of currently pregnant patients admitted with viral pneumonia from 2017-19: clearly shows that of 776 female Covid-19 patients admitted to ICU between the ages of 16 and 49, then in comparative terms, the good news is that currently pregnant women are 50% less likely to be admitted to ICU with Covid-19 than they are with viral pneumonia or influenza like illnesses. Men on the other hand are 142% more likely to be admitted to ICU with Covid-19 than viral pneumonia.

It should also be noted that “233 (56%) pregnant women admitted to hospital with SARS-CoV-2 infection in pregnancy were from black or other ethnic minority groups, 281 (69%) were overweight or obese, 175 (41%) were aged 35 or over, and 145 (34%) had pre-existing comorbidities.”
Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study
In conclusion we have a bio-chemical explanation of how folic acid impedes Covid-19 from binding to ACE2 receptors as a first line of defence. We also have evidence in a randomised control trial of the general public who were given Folate as a placebo and hospitalizations fell to zero, below the study designers expectations. Now we also have data on how pregnant women in their first trimester seem to be virtually unaffected by COVID-19: all of which collectively suggest that both HCQ and Folate/Folic Acid are important, cheap and safe therapeutic agents which are worthy of deeper medical research and immediate addition to integrated Covid-19 treatment protocols.

