I'm going to admit, this subject still confuses me and in these discussions I get the feeling I'm not on rock solid ground in terms of understanding. It is complex to go down to the nuts and bolts of it and I end up simply lost as I read deeper into it. I end up simply skimming the article and just going past it towards the summary. Because of this, I feel like I've not mastered grade school before going to high school when it comes to this. So I'm going to just explain my understanding of it and if you see anything wrong, please correct me.
1. Oxidative metabolism is the most efficient way of producing energy for humans. It involves the use of oxygen and glucose to produce energy, with carbon dioxide as one of the by-products. It is also called by another names, which at this moment eludes me. The mitochondria of the cell is where this takes place.
2. Aerobic glycolysis is a less efficient way of producing energy. Oxygen and glucose is also involved, but for some reason, there is xomething missing that keeps oxidative metabolism from being the energy production pathway. What is the reason? Is a by-product here lactic acid or carbon dioxide?
3. Anaerobic glycolysis uses glucose but no oxygen. This happens when there is a lack of oxygen, and the body resorts to this pathway. It involves fermentation and lactic acid is a by-product.
4. When the body is low on sugar, adrenaline and glucagon signals the liver to convert glycogen to glucose. When there is enough glycogen, does adrenaline cause cortisol also be produced?
5. When the blood sugar is low (hypoglycemia), the adrenal gland produces the hormone cortisol, and this converts protein, using certain amino acids in the protein to make glucose, in a process called gluconeogenesis.
There are also other amino acids in protein which can be converted into fat, what happens to this fat? Is it stored or is it metabolized?
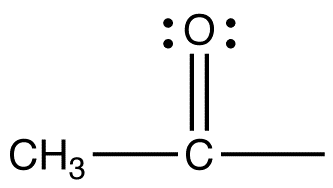
6. How is fat metabolized? Does it get converted also to glucose and metabolized? Or is fat metabolized as fat? What is the difference, energy-wise and by-product-wise, when fat is metabolized?
7. What is the difference between saturated fat being metabolized and PUFAs being metabolized?
8. What is the difference between short & medium chain fatty acid metabolism and long chain fatty acid metabolism?
9. Why does coconut oil bypass the liver and gets metabolized directly by cells, if that is true at all? Is it because it is a saturated oil? Or is because it is saturated and has short and medium chain fatty acids? Does the oil from butter bypass the liver as well? What about PUFAs that are short and medium chained, will it bypass the liver?
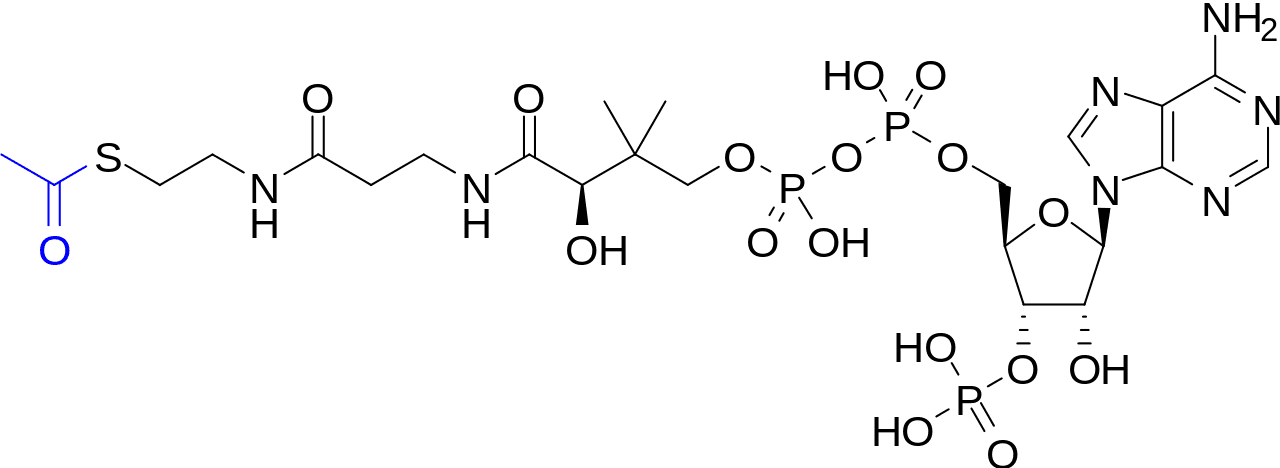
I admit to getting lost when the discussion turns into equations of acetyl-CoA, pyruvates, acetyl acetates. The chain of reactions involved needs me to microdose on LSD to fully understand it (I hope?). If you could explain in dummy terms, that would be great.
And I've tried doing some research. It just doesn't stick. Or maybe I never got to hit the bulls-eye. But in the discussions on cancer, and whether sugar helps the healing more than it helps the cancer, without understanding this well enough, it's very hard to sift through the confusion.
Hope someone can clear this up for me, and certainly I hope there is a unified thought on this between mainstream science and alternative science. Or will there still be major points of disagreement. It doesn't appear to be so, and probably the best test is if all Wikipedia entries on energy metabolism find agreement in this forum.
1. Oxidative metabolism is the most efficient way of producing energy for humans. It involves the use of oxygen and glucose to produce energy, with carbon dioxide as one of the by-products. It is also called by another names, which at this moment eludes me. The mitochondria of the cell is where this takes place.
2. Aerobic glycolysis is a less efficient way of producing energy. Oxygen and glucose is also involved, but for some reason, there is xomething missing that keeps oxidative metabolism from being the energy production pathway. What is the reason? Is a by-product here lactic acid or carbon dioxide?
3. Anaerobic glycolysis uses glucose but no oxygen. This happens when there is a lack of oxygen, and the body resorts to this pathway. It involves fermentation and lactic acid is a by-product.
4. When the body is low on sugar, adrenaline and glucagon signals the liver to convert glycogen to glucose. When there is enough glycogen, does adrenaline cause cortisol also be produced?
5. When the blood sugar is low (hypoglycemia), the adrenal gland produces the hormone cortisol, and this converts protein, using certain amino acids in the protein to make glucose, in a process called gluconeogenesis.
There are also other amino acids in protein which can be converted into fat, what happens to this fat? Is it stored or is it metabolized?
6. How is fat metabolized? Does it get converted also to glucose and metabolized? Or is fat metabolized as fat? What is the difference, energy-wise and by-product-wise, when fat is metabolized?
7. What is the difference between saturated fat being metabolized and PUFAs being metabolized?
8. What is the difference between short & medium chain fatty acid metabolism and long chain fatty acid metabolism?
9. Why does coconut oil bypass the liver and gets metabolized directly by cells, if that is true at all? Is it because it is a saturated oil? Or is because it is saturated and has short and medium chain fatty acids? Does the oil from butter bypass the liver as well? What about PUFAs that are short and medium chained, will it bypass the liver?
I admit to getting lost when the discussion turns into equations of acetyl-CoA, pyruvates, acetyl acetates. The chain of reactions involved needs me to microdose on LSD to fully understand it (I hope?). If you could explain in dummy terms, that would be great.
And I've tried doing some research. It just doesn't stick. Or maybe I never got to hit the bulls-eye. But in the discussions on cancer, and whether sugar helps the healing more than it helps the cancer, without understanding this well enough, it's very hard to sift through the confusion.
Hope someone can clear this up for me, and certainly I hope there is a unified thought on this between mainstream science and alternative science. Or will there still be major points of disagreement. It doesn't appear to be so, and probably the best test is if all Wikipedia entries on energy metabolism find agreement in this forum.