Supplemental oxygen administration is frequently used in premature infants and adults with pulmonary insufficiency. NADPH quinone oxidoreductase (NQO1) protects cells from oxidative injury by decreasing reactive oxygen species (ROS). In this investigation, we tested the hypothesis that overexpression of NQO1 in BEAS-2B cells will mitigate cell injury and oxidative DNA damage caused by hyperoxia and that A-1221C single nucleotide polymorphism (SNP) in the NQO1 promoter would display altered susceptibility to hyperoxia-mediated toxicity. Using stable transfected BEAS-2B cells, we demonstrated that hyperoxia decreased cell viability in control cells (Ctr), but this effect was differentially mitigated in cells overexpressing NQO1 under the regulation of the CMV viral promoter, the wild-type NQO1 promoter (NQO1-NQO1), or the NQO1 promoter carrying the SNP. Interestingly, hyperoxia decreased the formation of bulky oxidative DNA adducts or 8-hydroxy-2′-deoxyguanosine (8-OHdG) in Ctr cells. qPCR studies showed that mRNA levels of CYP1A1 and NQO1 were inversely related to DNA adduct formation, suggesting the protective role of these enzymes against oxidative DNA injury. In SiRNA experiments entailing the NQO1-NQO1 promoter, hyperoxia caused decreased cell viability, and this effect was potentiated in cells treated with CYP1A1 siRNA. We also found that hyperoxia caused a marked induction of DNA repair genes DDB2 and XPC in Ctr cells, supporting the idea that hyperoxia in part caused attenuation of bulky oxidative DNA lesions by enhancing nucleotide excision repair (NER) pathways. In summary, our data support a protective role for human NQO1 against oxygen-mediated toxicity and oxidative DNA lesions in human pulmonary cells, and protection against toxicity was partially lost in SNP cells. Moreover, we also demonstrate a novel protective role for CYP1A1 in the attenuation of oxidative cells and DNA injury. Future studies on the mechanisms of attenuation of oxidative injury by NQO1 should help in developing novel approaches for the prevention/treatment of ARDS in humans.

 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
Supplemental oxygen is an integral part of medical management of pediatric and adult patients with pulmonary insufficiency [1–3]. In premature infants and adults, exposure to hyperoxia contributes to the development of bronchopulmonary dysplasia (BPD) [4, 5], and in adults, it could exacerbate acute respiratory distress syndrome (ARDS) [6–8]. ARDS is a life-threatening illness that affects up to 10% of patients in intensive care units worldwide [9] and could develop following pneumonia, nonpulmonary sepsis, trauma, or aspiration [9]. Despite significant medical advances, mortality due to ARDS is high (35-46%) [8, 9], and recent studies have shown that ARDS is one of the major causes of death due to the COVID-19 infection [10]. The molecular mechanisms of oxygen-mediated lung injury are not completely understood, but reactive oxygen species (ROS) likely play an important role [11]. Hyperoxia (>95% FiO2) for 72 hours in rodents results in lung inflammation and injury, eventually leading to cell death [4, 12]. ROS generated in hyperoxic conditions lead to profound cell damage through direct DNA damage, lipid peroxidation, protein oxidation, and alteration of transcription factors [4, 12]. Recent studies from our laboratory have shown a protective effect of cytochrome P450 (CYP) 1A enzymes against hyperoxic lung injury in vivo [13–20].
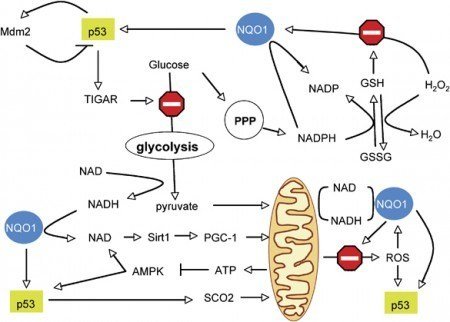
NADPH quinone oxidoreductase 1 (NQO1) is a phase II enzyme whose activity in the cell is to catalyze the two-electron reduction of quinone compounds, which prevents the generation of ROS and, thus, protects cells from oxidative damage [21]. Das et al. showed that mice deficient in the genes for Nqo1 and Nqo2 are more susceptible to lung injury than wild-type mice [22]. A number of single nucleotide polymorphisms (SNPs) have been reported for NQO1 [23–28]. Although associations between genetic variants in NQO1 and ALI/ARDS have been reported [22–28], little is known regarding the mechanisms by which these genetic variants contribute to ARDS.
In summary, our data support a protective role for human NQO1 against oxygen-mediated toxicity and oxidative DNA lesions in human pulmonary cells, and this protection is partially lost in cells carrying the A-1221C SNP. Moreover, we also demonstrate a novel protective role for CYP1A1 in the attenuation of oxidative cell and DNA injury. Future studies on the mechanisms of attenuation of oxidative injury by NQO1 should help in developing novel approaches for the prevention/treatment of ARDS in humans.

Role of Human NADPH Quinone Oxidoreductase (NQO1) in Oxygen-Mediated Cellular Injury and Oxidative DNA Damage in Human Pulmonary Cells
Supplemental oxygen administration is frequently used in premature infants and adults with pulmonary insufficiency. NADPH quinone oxidoreductase (NQO1) protects cells from oxidative injury by decreasing reactive oxygen species (ROS). In this investigation, ...
Supplemental oxygen is an integral part of medical management of pediatric and adult patients with pulmonary insufficiency [1–3]. In premature infants and adults, exposure to hyperoxia contributes to the development of bronchopulmonary dysplasia (BPD) [4, 5], and in adults, it could exacerbate acute respiratory distress syndrome (ARDS) [6–8]. ARDS is a life-threatening illness that affects up to 10% of patients in intensive care units worldwide [9] and could develop following pneumonia, nonpulmonary sepsis, trauma, or aspiration [9]. Despite significant medical advances, mortality due to ARDS is high (35-46%) [8, 9], and recent studies have shown that ARDS is one of the major causes of death due to the COVID-19 infection [10]. The molecular mechanisms of oxygen-mediated lung injury are not completely understood, but reactive oxygen species (ROS) likely play an important role [11]. Hyperoxia (>95% FiO2) for 72 hours in rodents results in lung inflammation and injury, eventually leading to cell death [4, 12]. ROS generated in hyperoxic conditions lead to profound cell damage through direct DNA damage, lipid peroxidation, protein oxidation, and alteration of transcription factors [4, 12]. Recent studies from our laboratory have shown a protective effect of cytochrome P450 (CYP) 1A enzymes against hyperoxic lung injury in vivo [13–20].
NADPH quinone oxidoreductase 1 (NQO1) is a phase II enzyme whose activity in the cell is to catalyze the two-electron reduction of quinone compounds, which prevents the generation of ROS and, thus, protects cells from oxidative damage [21]. Das et al. showed that mice deficient in the genes for Nqo1 and Nqo2 are more susceptible to lung injury than wild-type mice [22]. A number of single nucleotide polymorphisms (SNPs) have been reported for NQO1 [23–28]. Although associations between genetic variants in NQO1 and ALI/ARDS have been reported [22–28], little is known regarding the mechanisms by which these genetic variants contribute to ARDS.
In summary, our data support a protective role for human NQO1 against oxygen-mediated toxicity and oxidative DNA lesions in human pulmonary cells, and this protection is partially lost in cells carrying the A-1221C SNP. Moreover, we also demonstrate a novel protective role for CYP1A1 in the attenuation of oxidative cell and DNA injury. Future studies on the mechanisms of attenuation of oxidative injury by NQO1 should help in developing novel approaches for the prevention/treatment of ARDS in humans.