Funnywhensunny
Member
- Joined
- Feb 15, 2018
- Messages
- 313
Thank you for keeping us posted on this stuff, Pina.
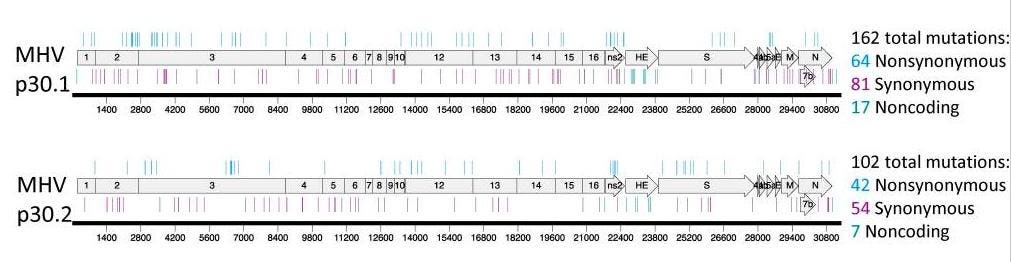
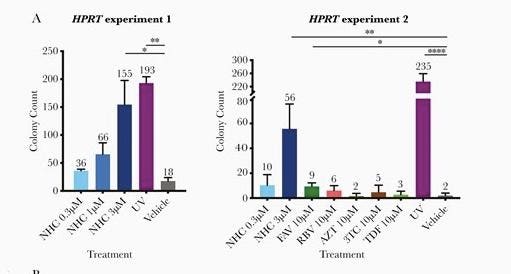
NY Times Casually Admits New FDA-Approved Merck COVID Drug Might Actually Mutate Healthy Human DNA by Accident and Impact Male Fertility | The Gateway Pundit | by Jim Hoft
In normal times, in a normal world, this news might make headlines.www.thegatewaypundit.com