SAFarmer
Member
- Joined
- Jul 31, 2013
- Messages
- 182
I did not say my equation is more than normal. I said the true theoretical equation gives an even higher value for ATP production.Suikerbuik said:SAFarmer, these are values corrected for cellular activities, in my opinion this is not more than normal.
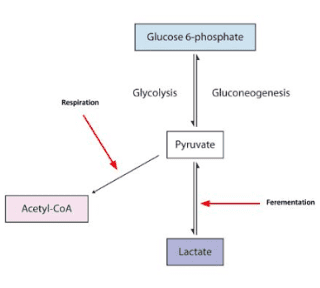
I did say for fully metabolised, didn't I ?Suikerbuik said:What your equation is saying is that that for 1 mole or molecule of glucose you produce 6 moles or molecules CO2 (ratio 1:6). You are correct about these CHO's can't disappear or form out of nowhere. However the formation of 6CO2, 6H2O and 30ATP is not a given certainty for every molecule of glucose you ingest ONLY when it's fully oxidized.
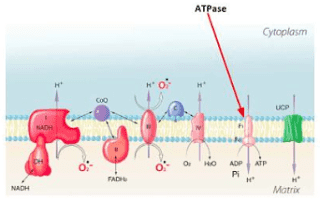
This is my point and question. For someone able to give me an example (with balanced equations) and practical explanation of how and what happens during "uncoupling" ito ATP , heat and other products.Suikerbuik said:When uncoupling happens for example you produce less ATP, so also less H2O is formed (unless uncoupling proteins also form H2O, but I didn'' dug into this).
This I agree and said as much. I just would like to see an example.Suikerbuik said:So the ATP is variable as is H2O and heat formation.
I dont think this was ever in dispute and not the issue of debate.Suikerbuik said:Also in practice part of your glucose intake will be converted into lactid acid (in most people). Lactid acid can be converted back to glucose (costs ATP) but also glycogen or protein. Also intermediates from the metabolism of glucose can be converted into amino acids or excreted. Acetyl CoA can be used for the formation of fatty acids (costs ATP). Glucose can be shunted in the pentose phosphate pathway. Probably there are more pathways I can't think of now.