Interesting. An IC would definitely not suit your purpose. Thanks for explaining what you are working toward in more detail.visionofstrength said:Yes, good example. Those directly measure CO2 and O2 but display the conventional "caloric" calculation, as described in the standard theory of RQ.Blossom said:I wonder if an indirect calorimeter could help?
In my view, if Peat is right about hypoxia and uncoupling, the calorimeters are not calculating the "calories" the way they think, because the RQ standard theory ignores the effects of hypoxia or uncoupling (which don't follow the RQ standard theory about calories).
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
-
By using this site you agree to the terms, rules, and privacy policy.
-
Charlie's Restoration Giveaway #2 (Entire Home EMF Mitigation & Protection Along With Personal Protection) - Click Here To Enter
-
Dear Carnivore Dieters, A Muscle Meat Only Diet is Extremely Healing Because it is a Low "vitamin A" Diet. This is Why it Works so Well...
Rest the rest of this post by clicking here
-
The Forum is transitioning to a subscription-based membership model - Click Here To Read
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Optimal Diet For Increasing Lifespan
- Thread starter haidut
- Start date
SAFarmer
Member
- Joined
- Jul 31, 2013
- Messages
- 182
visionofstrength said:I'm may be drawing a distinction perhaps between theory and practice.
In practice, you directly measure CO2 eliminated and O2 consumed, using devices that measure these things. RQ is the ratio of these two direct measurements.
In theory taught in university (or any theory even as summarized on wikipedia), RQ can be deduced without direct measurement. See for example this online calculator:
http://home.fuse.net/clymer/rq/
But these theoretical deductions, without direct measurement, are only true in theory, or ex vivo. They do not explain what you directly measure in practice, because in practice you are directly measuring the effects of hypoxia and uncoupling.
It's just something you observe when you do direct measurements, you find that RQ does not come out as the theory predicts, and then Peat's (and even Buteyko's) ideas about hypoxia and uncoupling help explain what you observe and directly measure.
No, I still think you are mistaken or not looking at the right things.
What you linked to is just a theoretic R/Q of a chemical formula being "burned" or metabolised . There is no "theory" or equation of what one's (humans) RQ value should be. Everyone is different and it changes according to health status and exercise or level of activity. It can only be measured like you said by O2 and CO2 levels. But RQ only tells you what the RELATIVE amounts of glucose vs fat is thats being metabolised, NOT the actual RATE or absolute value of metabolism. For that you need to measure the amount of O2 being used together with RQ and you possibly need to measure heat produced as well which probably can only be accurately be measured in a metabolic chamber. Indirect Calori measurement is an approximation of metabolic rate.
visionofstrength
Member
I think we are understanding each other about everything except perhaps for this:SAFarmer said:visionofstrength said:I'm may be drawing a distinction perhaps between theory and practice.
In practice, you directly measure CO2 eliminated and O2 consumed, using devices that measure these things. RQ is the ratio of these two direct measurements.
In theory taught in university (or any theory even as summarized on wikipedia), RQ can be deduced without direct measurement. See for example this online calculator:
http://home.fuse.net/clymer/rq/
But these theoretical deductions, without direct measurement, are only true in theory, or ex vivo. They do not explain what you directly measure in practice, because in practice you are directly measuring the effects of hypoxia and uncoupling.
It's just something you observe when you do direct measurements, you find that RQ does not come out as the theory predicts, and then Peat's (and even Buteyko's) ideas about hypoxia and uncoupling help explain what you observe and directly measure.
No, I still think you are mistaken or not looking at the right things.
What you linked to is just a theoretic R/Q of a chemical formula being "burned" or metabolised . There is no "theory" or equation of what one's (humans) RQ value should be. Everyone is different and it changes according to health status and exercise or level of activity. It can only be measured like you said by O2 and CO2 levels. But RQ only tells you what the RELATIVE amounts of glucose vs fat is thats being metabolised, NOT the actual RATE or absolute value of metabolism. For that you need to measure the amount of O2 being used together with RQ and you possibly need to measure heat produced as well which probably can only be accurately be measured in a metabolic chamber. Indirect Caloric measurement is an approximation of metabolic rate.
"But RQ only tells you what the RELATIVE amounts of glucose vs fat is thats being metabolised"
RQ does claim to tell you that, and can tell you that ex vivo (or even in the calculator I linked to).
But in vivo it cannot possibly tell you that (in my view), because the standard theory behind it fails to acknowledge [Edit: (though you clearly do)] the importance of metabolic rate, which cannot be factored out, or assumed away, as the standard theory claims to do.
Here's a simplified example, illustrated with direct measurements (assuming sea level, low humidity, with an O2 partial pressure of 21%):
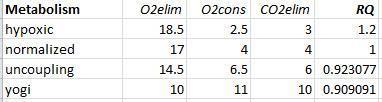
The differences shown here, resulting from metabolic rate, are too great to factor out, or assume away, and make calculating a relative value of glucose to fat --- from direct measurement -- too unreliable to be useful, I think. But standard theories are slow to catch up to actual data, if they ever do, and worse, the profound effect of uncoupling is still not accepted or even known by university graduates trained in RQ.
SAFarmer
Member
- Joined
- Jul 31, 2013
- Messages
- 182
VoS,
You give a table without any reference study or anything else which is meaningless without context.
You assume I fail to acknowledge "something" without stating exactly what. You still seem to think "theory" is extrapolating metabolic rate from an RQ value ?
I believe true metabolic rate can only be accurately measured with "real data" in a metabolic chamber. Do you disagree with this ?
You give a table without any reference study or anything else which is meaningless without context.
You assume I fail to acknowledge "something" without stating exactly what. You still seem to think "theory" is extrapolating metabolic rate from an RQ value ?
I believe true metabolic rate can only be accurately measured with "real data" in a metabolic chamber. Do you disagree with this ?
visionofstrength
Member
Sorry! I hope I meant to say that you do acknowledge the importance of metabolic rate, as you've said! [I edited the post to try to make this clearer.] I only wanted to explain that one small point: Standard theory is extrapolating relative values of glucose and fat from an RQ value. That's the part that cannot be so, in my view.SAFarmer said:VoS,
You give a table without any reference study or anything else which is meaningless without context.
You assume I fail to acknowledge "something" without stating exactly what. You still seem to think "theory" is extrapolating metabolic rate from an RQ value ?
I believe true metabolic rate can only be accurately measured with "real data" in a metabolic chamber. Do you disagree with this ?
I tried to point out that I simplified the table, simply to be illustrative, not authoritative. Sorry again!
(assuming sea level, low humidity, with an O2 partial pressure of 21%):

If studies might help, the Buteyko advocates rely on a number of them that they claim support this line of thinking, and Peat has discussed some in an interview about Buteyko, as I recall? Here's a page that describes Buteyko's own study, and claims there are many similar Western studies (though I haven't verified this).
From Peat's perspective, though, it's a little easier to describe: hypoxic people hyperventilate, blowing off CO2 and consuming little oxygen; while healthy people uncouple respiration from ATP, generating more CO2, and consuming more oxygen (via the Haldane effect).
The important thing (if only for me) is that, as I read Peat, to determine true metabolism, it's the degree of uncoupling (and CO2 generation) that matters. And if true, the implications are many and quite unexpected!
[Side note: I don't know much about metabolic chambers. Do they measure heat? Does heat equate to CO2 generation? (I haven't thought about it, and I don't use a heat chamber for my experiments, only CO2 sensors.)]
SAFarmer
Member
- Joined
- Jul 31, 2013
- Messages
- 182
VoS
I'll try to make my point, again, slowly ...
These are the equations for complete "burning" of glucose and fat :
Glucose:
C6H12O6 + 6O2 >> 12H2O + 6 CO2 + 30 ATP + heat
Palmitic acid (Sat fat)
C16H32O2 + 23 O2 >> 16H2O + 16 CO2 + 106 ATP + heat
So the higher one's metabolism the more glucose or fat is burned and more ATP, CO2 and heat is produced.
As far as your "decoupling" argument goes, I havent seen a lot of studies or evidence on this front, but from what I understand from Peat is that it means less ATP is produced and more Co2 and heat in it's place. But the equation still have to balance imo .
You will also now (hopefully) see that why when it's important to measure metabolic rate accurately, a metabolic chamber is the only place to do it where you can measure and account for heat transfer as well.
I'll try to make my point, again, slowly ...
These are the equations for complete "burning" of glucose and fat :
Glucose:
C6H12O6 + 6O2 >> 12H2O + 6 CO2 + 30 ATP + heat
Palmitic acid (Sat fat)
C16H32O2 + 23 O2 >> 16H2O + 16 CO2 + 106 ATP + heat
So the higher one's metabolism the more glucose or fat is burned and more ATP, CO2 and heat is produced.
As far as your "decoupling" argument goes, I havent seen a lot of studies or evidence on this front, but from what I understand from Peat is that it means less ATP is produced and more Co2 and heat in it's place. But the equation still have to balance imo .
You will also now (hopefully) see that why when it's important to measure metabolic rate accurately, a metabolic chamber is the only place to do it where you can measure and account for heat transfer as well.
visionofstrength
Member
These equations for burning glucose and fat have been demonstrated ex vivo, but in vivo the extrapolation from RQ of whether you are burning glucose or fat is nothing more than university dogma and can't be demonstrated, because uncoupling processes may be at work.SAFarmer said:VoS
I'll try to make my point, again, slowly ...
These are the equations for complete "burning" of glucose and fat :
Glucose:
C6H12O6 + 6O2 >> 12H2O + 6 CO2 + 30 ATP + heat
Palmitic acid (Sat fat)
C16H32O2 + 23 O2 >> 16H2O + 16 CO2 + 106 ATP + heat
So the higher one's metabolism the more glucose or fat is burned and more ATP, CO2 and heat is produced.
As far as your "decoupling" argument goes, I havent seen a lot of studies or evidence on this front, but from what I understand from Peat is that it means less ATP is produced and more Co2 and heat in it's place. But the equation still have to balance imo .
Here's the paper from Gilbert Ling that it seems first expressed the uncoupling equations:
http://www.physiologicalchemistryandphy ... 9_ling.pdf
[Minor point: I can see why it's important and practical to measure CO2 generation. But why is heat transfer also important to measure? If heat transfer can be inferred from CO2 generation during uncoupling, then why not just measure CO2 generation? Or are you saying that heat cannot be inferred from CO2 generation during uncoupling?SAFarmer said:You will also now (hopefully) see that why when it's important to measure metabolic rate accurately, a metabolic chamber is the only place to do it where you can measure and account for heat transfer as well.
Put another way: There are only 14 metabolic chambers in the US. If my experiment required a metabolic chamber, then I might think about designing another experiment! Restricted access to proprietary devices is how the corrupt patrinomy of US academicians avoids open dialogue and critique.
Now if I was lucky enough to be in South Africa, I'd have an open source metabolic chamber!
http://www.techcentral.co.za/sas-open-s ... ber/36779/]
SAFarmer
Member
- Joined
- Jul 31, 2013
- Messages
- 182
Yes, we have an open source metabolic chamber in SA. Something we are the world ahead in !
I actually intend to visit them one day to measure my own metabolic rate.
But that's beside the point we are discussing here. Why don't you explain in your own words what you understand by "uncoupling" "in vivo" and how you think that works, ie where do the Carbon molecules come from, where do it go, what happens with ATP, heat, etc ? In the end, you can't get past the laws of nature, ex vivo or in vivo, all energy needs to be accounted for.
I actually intend to visit them one day to measure my own metabolic rate.
But that's beside the point we are discussing here. Why don't you explain in your own words what you understand by "uncoupling" "in vivo" and how you think that works, ie where do the Carbon molecules come from, where do it go, what happens with ATP, heat, etc ? In the end, you can't get past the laws of nature, ex vivo or in vivo, all energy needs to be accounted for.
Suikerbuik
Member
- Joined
- Jan 25, 2014
- Messages
- 700
Glucose:
C6H12O6 + 6O2 >> 12H2O + 6 CO2 + 30 ATP + heat
Palmitic acid (Sat fat)
C16H32O2 + 23 O2 >> 16H2O + 16 CO2 + 106 ATP + heat
Not sure what all this discussion is about to be honest. Just keep in mind that these are pure theoretical values.
visionofstrength
Member
That's what I'm sort of asking for ideas about, an understanding of Gilbert Ling's (and Peat's) ideas about uncoupling? It is, in my view, a still largely ignored phenomenon.SAFarmer said:Yes, we have an open source metabolic chamber in SA. Something we are the world ahead in !
I actually intend to visit them one day to measure my own metabolic rate.
But that's beside the point we are discussing here. Why don't you explain in your own words what you understand by "uncoupling" "in vivo" and how you think that works, ie where do the Carbon molecules come from, where do it go, what happens with ATP, heat, etc ? In the end, you can't get past the laws of nature, ex vivo or in vivo, all energy needs to be accounted for.
But I do feel strongly, based on my own experiments using CO2 sensors, that the textbook model of extrapolating from RQ theoretical values for burning glucose and fat can't possibly be right, or even useful in experiments. RQ is much more than that, and Ling's (and Peat's) description of uncoupling is what's actually behind RQ.
visionofstrength
Member
Hi S! Have you ever had a chance to study Ling's paper?Suikerbuik said:Glucose:
C6H12O6 + 6O2 >> 12H2O + 6 CO2 + 30 ATP + heat
Palmitic acid (Sat fat)
C16H32O2 + 23 O2 >> 16H2O + 16 CO2 + 106 ATP + heat
Not sure what all this discussion is about to be honest. Just keep in mind that these are pure theoretical values.
http://www.physiologicalchemistryandphy ... 9_ling.pdf
My chemistry background is a little weak, so if you can shed any light on Ling, please share?
Thanks!
Suikerbuik
Member
- Joined
- Jan 25, 2014
- Messages
- 700
I have Ling's book, but didn't read it completely yet. This paper looks very interesting and will take a better look at this some day (if it's not all discussed in Ling's book). Too bussy lately. Hopefully will have some time to look at it more closely in the weekend.
The conventional theory is that uncoupling is a process that reduces the proton concentration difference at the inner mitochondrial membrane. This concentration normally drives ATPase (at this step O- and 2H+ form H2O.). With uncoupling substances you manage to keep your electron transport chain going more easily without producing ATP. So you have a relative increase of carbon dioxide caused by uncoupling substances, because ATP and NADH inhibit several important enzymes in these pathways including pyruvate dehydrogenase and citrate synthase. All the carbon dioxide is mainly formed in the citric acid cycle. In other words by keeping your ATP and NADH at sufficient but not depleted levels there won't be a brake somewhere.
Edit: hopefully it's more clear now, as I said it quite compromised.
The conventional theory is that uncoupling is a process that reduces the proton concentration difference at the inner mitochondrial membrane. This concentration normally drives ATPase (at this step O- and 2H+ form H2O.). With uncoupling substances you manage to keep your electron transport chain going more easily without producing ATP. So you have a relative increase of carbon dioxide caused by uncoupling substances, because ATP and NADH inhibit several important enzymes in these pathways including pyruvate dehydrogenase and citrate synthase. All the carbon dioxide is mainly formed in the citric acid cycle. In other words by keeping your ATP and NADH at sufficient but not depleted levels there won't be a brake somewhere.
Edit: hopefully it's more clear now, as I said it quite compromised.
visionofstrength
Member
By conventional theory, is that the non-Ling cellular biology that talks about things like mitochondrial membranes?Suikerbuik said:The conventional theory is that ...
I'm just getting introduced to this, but from what I have seen so far, Ling seems to propose a cellular biology based on the "Association-Induction" process (not ion-impermeable membranes), and ATP is a "cardinal adsorbent" in that process.
SAFarmer
Member
- Joined
- Jul 31, 2013
- Messages
- 182
You guys are getting way offline here. Ling's AI hypothesis has got what to do with rate of metabolism and RQ values ?
I am still waiting for VoS to explain his reasoning with examples of measurement to show what he is talking about.
I am still waiting for VoS to explain his reasoning with examples of measurement to show what he is talking about.
SAFarmer
Member
- Joined
- Jul 31, 2013
- Messages
- 182
Exactly !visionofstrength said:By conventional theory, is that the non-Ling cellular biology that talks about things like mitochondrial membranes?Suikerbuik said:The conventional theory is that ...
I'm just getting introduced to this, but from what I have seen so far, Ling seems to propose a cellular biology based on the "Association-Induction" process (not ion-impermeable membranes), and ATP is a "cardinal adsorbent" in that process.
And how does this fit in with the rate of metabolism and Co2 produced as product of that ?
SAFarmer
Member
- Joined
- Jul 31, 2013
- Messages
- 182
Suikerbuik said:Glucose:
C6H12O6 + 6O2 >> 12H2O + 6 CO2 + 30 ATP + heat
Palmitic acid (Sat fat)
C16H32O2 + 23 O2 >> 16H2O + 16 CO2 + 106 ATP + heat
Not sure what all this discussion is about to be honest. Just keep in mind that these are pure theoretical values.
In fact, the ATP values in this equation is more real world vs pure theoretical, which is higher.
The point is you start from a "theoretical" proposition and then explain any real world differences there might be. But in the end you have to account for what happens with your C's H's and your O's ...
visionofstrength
Member
I'm hearing that I need to read Cells, Gels and the Engines of Life, by Pollack. But basically, it works something like this:SAFarmer said:Exactly !visionofstrength said:By conventional theory, is that the non-Ling cellular biology that talks about things like mitochondrial membranes?Suikerbuik said:The conventional theory is that ...
I'm just getting introduced to this, but from what I have seen so far, Ling seems to propose a cellular biology based on the "Association-Induction" process (not ion-impermeable membranes), and ATP is a "cardinal adsorbent" in that process.
And how does this fit in with the rate of metabolism and Co2 produced as product of that ?
The Association-Induction hypothesis states that ion exclusion in the cell is maintained by the structural ordering of water within the cytoplasm, by an interaction between the cytoskeletal proteins, water molecules, and ATP. Energy (in the form of ATP) is used to unfold proteins, presenting a regular pattern of surface charges to cell water. This orders the cell water into a 'gel like' phase which excludes specific ions, because their presence within the structure is energetically unfavorable. Other ions are selectively retained, because they are adsorbed to charged sites on protein surfaces. This structured state can be maintained with no additional energy.
Here's a diagram:
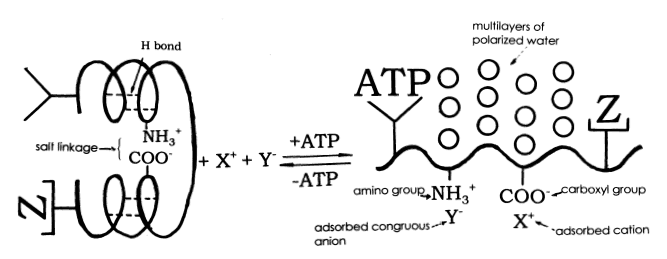
Is it OK if I fill you in as I learn? Thanks for asking! You've fired me up to tackle this. We all thrive on interaction.
visionofstrength
Member
[align=justify][/align]
This seems to be the best reference I can find from Peat's work:
visionofstrength said:I'm hearing that I need to read Cells, Gels and the Engines of Life, by Pollack. But basically, it works something like this:
The Association-Induction hypothesis states that ion exclusion in the cell is maintained by the structural ordering of water within the cytoplasm, by an interaction between the cytoskeletal proteins, water molecules, and ATP. Energy (in the form of ATP) is used to unfold proteins, presenting a regular pattern of surface charges to cell water. This orders the cell water into a 'gel like' phase which excludes specific ions, because their presence within the structure is energetically unfavorable. Other ions are selectively retained, because they are adsorbed to charged sites on protein surfaces. This structured state can be maintained with no additional energy.
Here's a diagram:

Is it OK if I fill you in as I learn? Thanks for asking! You've fired me up to tackle this. We all thrive on interaction.
This seems to be the best reference I can find from Peat's work:
Ray Peat said:Considering the universal importance of carbon dioxide to life, the ways it interacts with all of the important substances that make up organisms, that it is involved closely with ATP synthesis and other "energy-related" processes, that it participates intimately in the regulation of water and ions, that it is therapeutic in a range of conditions including angina pectoris, hypoxia, epilepsy, inflammation, shock, lipid peroxidation, pneumonia, and asthma, I think we can at least conclude that it is a largely overlooked mediator between chemical energy and life processes. In many cases, its movements and reactions constitute the actual motive force that so many fantasy theories have failed to explain. In other situations, it fills out the context for understanding the energy-mediating actions of ATP, calcium, and hormones.
In the special arrangement of matter that is the living state, in which the most common events involve processes that are so close to equilibrium that some of them can be thought of as oscillations in an elastic system, carbon dioxide participates in both enzymic and nonenzymic reactions that produce, conserve, transfer, and transform energy. In its quickly reversible binding to protein amino groups, for example, it alters the protein's electrical charge, its folding, and its manner of associating with water and other substances. Its availablility to occupy these groups protects them from attack by substances that would degrade the organism's energy and structure. If the protein, water, ionic system is thought of as energized matter, like a wound-up watch spring, it is the formation of carbon dioxide which has energized it and stabilized it.
Such_Saturation
Member
- Joined
- Nov 26, 2013
- Messages
- 7,370
Ray Peat wrote:
I had similar symptoms, I often ate several thousand calories per day without getting fat, and small noises would shock me awake. Taking thyroid reduced my caloric requirement, >and immediately allowed me to sleep deeply. Deficiencies of magnesium, vitamin A, and selenium probably contribute to that metabolic pattern.
(I got this on Toxinless)
I had similar symptoms, I often ate several thousand calories per day without getting fat, and small noises would shock me awake. Taking thyroid reduced my caloric requirement, >and immediately allowed me to sleep deeply. Deficiencies of magnesium, vitamin A, and selenium probably contribute to that metabolic pattern.
(I got this on Toxinless)
Suikerbuik
Member
- Joined
- Jan 25, 2014
- Messages
- 700
VOS, With conventional I indeed mean what is currently being taught. Altough the cellular mechanisms may be point of discussion and differ (I didn't day anything about this) the concept of uncoupling stays the same.
SAFarmer, these are values corrected for cellular activities, in my opinion this is not more than normal. What your equation is saying is that that for 1 mole or molecule of glucose you produce 6 moles or molecules CO2 (ratio 1:6). You are correct about these CHO's can't disappear or form out of nowhere. However the formation of 6CO2, 6H2O and 30ATP is not a given certainty for every molecule of glucose you ingest ONLY when it's fully oxidized. When uncoupling happens for example you produce less ATP, so also less H2O is formed (unless uncoupling proteins also form H2O, but I didn'' dug into this). So the ATP is variable as is H2O and heat formation.
Also in practice part of your glucose intake will be converted into lactid acid (in most people). Lactid acid can be converted back to glucose (costs ATP) but also glycogen or protein. Also intermediates from the metabolism of glucose can be converted into amino acids or excreted. Acetyl CoA can be used for the formation of fatty acids (costs ATP). Glucose can be shunted in the pentose phosphate pathway. Probably there are more pathways I can't think of now.
SAFarmer, these are values corrected for cellular activities, in my opinion this is not more than normal. What your equation is saying is that that for 1 mole or molecule of glucose you produce 6 moles or molecules CO2 (ratio 1:6). You are correct about these CHO's can't disappear or form out of nowhere. However the formation of 6CO2, 6H2O and 30ATP is not a given certainty for every molecule of glucose you ingest ONLY when it's fully oxidized. When uncoupling happens for example you produce less ATP, so also less H2O is formed (unless uncoupling proteins also form H2O, but I didn'' dug into this). So the ATP is variable as is H2O and heat formation.
Also in practice part of your glucose intake will be converted into lactid acid (in most people). Lactid acid can be converted back to glucose (costs ATP) but also glycogen or protein. Also intermediates from the metabolism of glucose can be converted into amino acids or excreted. Acetyl CoA can be used for the formation of fatty acids (costs ATP). Glucose can be shunted in the pentose phosphate pathway. Probably there are more pathways I can't think of now.
EMF Mitigation - Flush Niacin - Big 5 Minerals
Similar threads
- Replies
- 7
- Views
- 8K
- Replies
- 44
- Views
- 20K
- Replies
- 7
- Views
- 3K
- Replies
- 14
- Views
- 6K
- Replies
- 92
- Views
- 27K
- Replies
- 11
- Views
- 3K
- Replies
- 20
- Views
- 7K
- Replies
- 21
- Views
- 9K
- Replies
- 148
- Views
- 29K
- Replies
- 3
- Views
- 3K
- Replies
- 91
- Views
- 19K
- Replies
- 13
- Views
- 9K
- Replies
- 27
- Views
- 4K
- Replies
- 13
- Views
- 6K
