Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
The cause of myopia is not difficult to understand. There are tons of data sitting right before our eyes that can elucidate the basic mechanism of these vision changes. By reading published clinical case reports, biological optics, enzyme kinetics, and analytical chemistry articles, the answer plainly reveals itself:
I. Paul Riordan Eva
Much insight can be gained through the observations of Paul Riordan Eva, who critically examined the data regarding myopia. He had published an excellent review article in 1982, shown below:
In which he makes sense of the long‐noted observations of hyperglycemia on refractive changes—the propensity of the lens to bend light, and only real way to explain myopia. Trying to explain myopia through any other way leads to a contradiction: When imagining myopia to simply have as its cause the inability to focus, or a problem in controlling the iris, leads nowhere fast. That idea immediately falls apart when it's realized that anyone with nearsightedness can focus just fine, with glasses, and can easily bring objects far away into focus. The great majority of myopic changes are not a problem with the iris or any other muscle.
So a change in the refractive index has always been the primary focus among scientists, since there's no other logical way to explain it. The lens refractive index can actually change very quickly with raised blood glucose levels: Here are some quotes from Paul Riordan Eva to give you an idea of just how quick and drastic these changes can occur:
II. Case Reports—Excerpts
III. History of the Observation
These observations are in fact common, and have been noted for over a century. The diabetic cataract is certainly a well‐recognized pathology, and the diabetic refractive changes are recognized as well. Paul Riordan Eva documents observations along these lines dating back over a century:
The premise of Paul Riordan Eva's article appears to be a reminder to opthalmologists that hyperglycemia actually produces hyperopia, not myopia as sometimes thought. This is an important point to make note of, since this fact will be needed both to explain myopia itself and to suggest ways to improve it.
The prevalence of myopic changes in hyperglycemia is fairly common, much more common than in non‐diabetic people. I think you'd be forced to agree that glucose plays a fundamental role in the refractive changes of the lens.
IV. The 'Explanation'
Glucose became so well‐known to cause refractive changes off the lens that theoreticians felt obliged to explain it. Back then, in the early twentieth century, osmotic forces seemed the only logical way for doing so.
But there are flaws with such a theory. An influx of water caused by the hygroscopic forces of glucose would be expected to increase refraction, but the opposite is seen. What followed was a period of Rube Goldberg‐like ad hoc explanations of considerable magnitude.
And in fact, aldose reductase inhibitors have been shown in many studies to prevent these refractive changes in rabbits. It can no longer be argued that osmotic forces play a role in determining the width, shape, or refractive index of the lens itself. What is necessary to explain the effect of glucose on the lens necessarily lies downstream of aldose reductase, and none of those products are any more hygroscopic than glucose itself.
V. Aldose Reductase
This enzyme is an offshoot off normal glucose metabolism, and is only really active with the overflow of glucose seen in hyperglycemia. Under this enzyme, glucose is reduced to sorbitol. While not a dangerous molecule in itself, it doesn't have the protective phosphate group as it should. Too much glucose in the extracellular fluid—largely under the action of aldose reductase—leads to high amounts of trioses, acetol, and methylglyoxal: a common finding in diabetic blood.
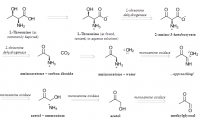
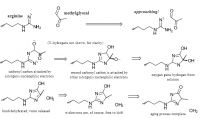
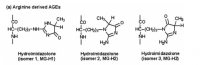
What is unique about methylglyoxal, among all things, is its ability to form a covalent adduct with arginine. This forms a cyclic imidazalone ring, which is fluorescent. This species is often referred to as 'MG‐H,' with subscipts denoting subtle variations. Although sometimes called an 'adduct,' this is misleading as it implies simple addition—not the transformation which actually occurs. The end result is actually an imidazalone, a fluorescent ring.
 reaction process depicted; click to embiggen
reaction process depicted; click to embiggen
(This ring is also sometimes called hydroimidazolone, but this isn't a very good name. The name that would make IUPAC the most happy would be Nᵟ‐(5‐hydro‐5‐methyl‐4‐imidazolon‐2‐yl)‐L‐ornithine. For short: it's better to accentuate the methyl group than the hydro and call it ornithyl‐methylimidazalone, or simply methylimidazalone. This name is used in publications found in the Journal of the American Chemical Society, so other people agree. The hydro is less conspicuous, and hence less deserving to be named. But of course, anyone can choose to name it after the much smaller hydrogen if they'd like. The point is: If anyone should decide to research this molecule, keep in mind that it goes by many names. It is even sometimes simply called an MG–arginine adduct. There's over six different names commonly used to describe this.)
Diabetic blood proteins are more fluorescent than normal people's for this reason, and taking L-arginine as a supplement has actually been used to counteract this. Such modifications are very well‐known among diabetes researchers.
The lens is about 90% protein, and roughly 10% carbohydrate. The primary protein of the lens is called crystallin, for obvious reasons. This protein can certainly be covalently modified by methylglyoxal, and acetol to a lesser—but still significant—extent. David Vander Jagt had shown this in 1992.
 γ-crystallin from uniprot.org (click to embiggen)
γ-crystallin from uniprot.org (click to embiggen)
The protein crystallin has many arginine residues, and thus would be expected to be covalently modified by methylglyoxal and acetol—products of the aldose reductase pathway.
VI. Proof of Imidazalone in the Lens
Though it may be fun to theorize about—analyze kinetic rates and blood glucose concentrations in attempt to estimate—we don't even have to. These covalent methylglyoxal and acetol adducts have been determined in the human lens in both hypoglycemic and euglycemic individuals.
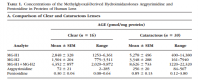
It was found, predictably, that the diabetics had more methylimidazalone rings on the crystallin proteins—which constitute around 90% of the lens.
At this point, things are becoming clear. The high glucose predictably leads to higher methylglyoxal and acetol concentrations, turning the arginine side‐chains of crystallin into fluorescent methylimidazalone side‐chains. Could this be expected to change the refractive index? I think so, and Naila Ahmed implies such a thing—giving us permission for thinking so (although we really don't need her permission).
 click to embiggen
click to embiggen
VII. The Molecular Refractive Index
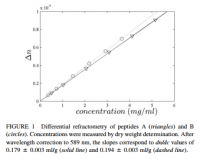
Proteins have a refractive index, usually given as the 'increment.' This increment is a ratio of the refractive index over the concentration, given as dn/dc. The 'n' denotes the Snell refractive index, and the 'c' indicates concentration (why it has units of ml/g). The 'd' indicates the derivative, but I'll use the partial differential symbol '∂' to accentuate this. The reason an increment is given, and the reason it's a fraction with unexpected units, is that it depends on the concentration. When an aqueous protein solution is put in a tube with light shining through, the refraction of light depends also on concentration. Luckily however, the graph of refraction over concentration is constant, and what is arrived at is a constant number: the refractive index increment, or ∂n/∂c.

 click to embiggen
click to embiggen
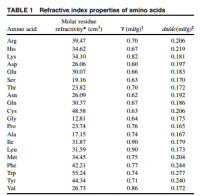
Different proteins have different refractive index increments, a value determined based only on the amino acids present. Since we're dealing with light, the amino acids tryptophan, tyrosine, and phenylalanine are of obvious significance.
Relevant to the topic of myopia: the refractive index increment of crystallin—the protein of the eye—is found to be higher than any other protein. What would you expect? This protein was designed for this of course:
The types of amino acids present dictate the refractive index. Protein folding has surprisingly little effect. So powerful is this difference in only the amino acids themselves, they alone can actually reliably predict the refractive index increment of an entire protein.
So the osmolarity of glucose solution isn't the only way to change the refractive index of the lens, even if that explanation could ever be made to make sense. All that needs to be done—based on scientific measurements and described by Huaying Zhao's equations—is to change the relative proportions of the amino acids of the protein, with special emphasis given to the aromatic and fluorescent amino acids. To explain the observations of Paul Riordan Eva—that hypergylcemia is characterized by hyperopia—all one needs to explain is the change in one amino acid, or the transformation of arginine to fluorescent methylimidazalone: A covalent product formed from arginine and either methylglyoxal or acetol. This is the very same thing you'd expect from hyperglycemia—an increase in methylimidazalone—and the very same chemical species found by Naila Ahmed in human diabetic lens protein.
An image focusing behind the retina would represent an increase in the refractive index increment (∂n/∂c) or the lens. The ∂n/∂c of tryptophan, tyrosine, phenylanine are the highest among the amino acids with values of 0.277, 0.240, and 0.244·ml/g, respectively (Zhao, Table 1). The ∂n/∂c of arginine is 0.206·ml/g, and is less than the aromatics. Since all aromatics—and histidine too—have a higher refractive index increment than those not cyclic, you'd expect methylimidazalone have a higher refractive index increment than arginine. For this reason, you would expect higher concentrations of methylglyoxal and acetol in the lens to increase the total refractive index and create hyperopia. This confirms all observations and leaves nothing wanting—with no paradox.
The osmotic hypothesis cannot explain why hyperopia would form suddenly, and then take weeks to reverse. An osmotic theory would be depending on glucose, in real time, and also be at the whims of sodium concentration—never considered to be a factor in refractive changes. The sudden onset in the above case observed by Paul Eva can be explained by the transformation of arginine into methylimidazalone in the lens protein crystallin, thereby increasing the refractive index of said protein. The gradual recovery can be explained by the relatively‐slow protein turnover rate.
VIII. Myopia
Looking back on the analytical determinations of Ahmed, you can see that both the normal lens and the cataract lens have methylimidazalone. The diabetic lens has more, but only about twice as much as the controls—victims of 'injury or trauma.' It would then appear that the lens is normally modified by the products of methylglyoxal and acetol as a result of glucose metabolism: The lens naturally has some degree of methylimidazalone in place of arginine. There is no amino acid one‐letter code for this, and you could never see it denoted in the protein sequence anyway because it's a post‐translational modification—you could never really predict which arginine would be modified. Nonetheless, they exist. These fluorescent species with higher ∂n/∂c are found in the normal human lens (Ahmed, Table 1).
Hypoglycemia would be expected to cause myopia by attenuating the amount of methylimidazalone formed. This would lower refractive index increment back to that of arginine, and lower the global refractive index of the entire lens. This would cause the light to bend less, dropping short of the retina.
You would not expect myopia to form as quickly as hyperopia since the reversion back to arginine requires new protein synthesis, in the relative absence of glucose.
IX. Metabolic Considerations
The sorbitol pathway of the lens has been thoroughly‐studied: Enzyme's have been analyzed; they have been homogenized and centrifuged, then precipitated and further refined through column chromatography. Two glycolytic enzymes found in the lens have been kinetically measured under a variety of substrates. These two enzymes, aldose reductase and polyol dehydrogenase, are dependent on their cofactor NADH. This means that either a paucity of niacin or a change in redox balance could theoretically change methylglyoxal concentrations, and subsequent changes in lens refractive index. Also, if present, glutathione working through glyoxylase I & II would be expected to play a role. Glutathione would be expected to lower methylglyoxal concentrations through the well‐characterized glyoxal cycle (see Thornally).
 click to embiggen
click to embiggen
X. Wikipedia is Wrong
Wikipedia is wrong, but that's only because I haven't yet gotten around to making the edit. Besides their wrongness in this particular case, they are also quite wrong about many other things.
XI. Miscellaneous Quotes
"Experimental data suggest that glucose diffuses into the lens and permeates the small extracellular space, which accounts for 5-10% of the lens volume," ―Paul Riordan Eva
"However, if glucose levels in the surrounding medium are increased, lens fibres seem to accumulate glucose [and produce methylglyoxal], and more especially-because of the apparent diversion of this substrate into the sorbitol pathway the metabolites sorbitol and fructose [and methylglyoxal and acetol]." ―Paul Riordan Eva
"The reason is probably saturation of the enzyme hexokinase, the starting point of the glycolytic and pentose phosphate pathways in combination with the markedly higher Km of aldose reductase, the first enzyme of the sorbitol pathway." ―Paul Riordan Eva
"The net result is an accumulation of fructose and sorbitol [and methylglyoxal and acetol downstream of that], both of which penetrate lens membranes poorly and are further metabolised to only a limited extent." ―Paul Riordan Eva
"Experiments with human lenses have shown that lens tissue is capable of forming sufficiently high levels of sorbitol [and methylglyoxal] with 24 hours of continuous hyperglycaemia," ―Paul Riordan Eva
"It would thus appear that a decrease in the refractive indices within the anterior portions of the lens might produce the hyperopia associated with hyperglycaemia." ―Paul Riordan Eva
"Recently, we have found similar high concentrations of MG-H₁ in protein extracts of human blood cells and tissues of laboratory rats." ―Naila Ahmed
"The formation of argpyrimidine is favored by high concentrations of methylglyoxal and oxidative processes." ―Naila Ahmed
"The activity of glyoxalase I and the concentration of triosephosphates are important variables controlling methylglyoxal concentration and related glycation in cultured rat lens." ―Naila Ahmed
"In this report, we found that MG-H was indeed the major methylglyoxal-derived AGE and that it was present at high concentration." ―Naila Ahmed
"The availability of accurate protein refractive indices is also crucial for understanding the biophysics of eyes. For example, dn/dc enters as a key parameter in models for light transmission and scattering in the cornea. Similarly, lens protein concentrations are often estimated from measured lens refractive indices on the basis of assumed crystallin dn/dc values." ―Huaying Zhao
"In a forthcoming communication, we show by systematic sequence analysis of crystallins of different members of the crystallin family that lens crystallins have indeed specifically evolved toward an elevated refractive index increment." ―Huaying Zhao
"As a consequence, the protein amino acid composition represents the major determinant for the protein refractive index increment, dn/dc." ―Huaying Zhao
"Physically, the refractive index of particles in the visible spectrum of light is a result of the local polarizability of the atoms and chemical groups due to deformation of the electron configuration about nuclei, and therefore insensitive to the long-range structure of macromolecules, and long known to be to a good approximation additive toward macromolecular refractivity." ―Huaying Zhao
"For a spherical lens, in which the refractive index distribution depends only on the distance from the lens centre, equations for the paths of rays through the lens can be derived from a knowledge of the refractive index distribution within the lens (Born and Wolf, 1970)." ―Melanie C.W.Campbell
XII. References
Bloemendal, Hans. "Lens protein." Critical Reviews in Biochemistry (1982)
Zhao, Huaying. "On the distribution of protein refractive index increments." Biophysical journal (2011)
Campbell, Melanie. "Measurement of refractive index in an intact crystalline lens." Vision Research (1984)
Eva, Paul Riordan. "Refractive change in hyperglycaemia: hyperopia, not myopia." British Journal of Ophthalmology (1982)
Jedziniak, Judith A. "The sorbitol pathway in the human lens: aldose reductase and polyol dehydrogenase." Invest Ophthalmol Vis Sci (1981)
Ahmed, Naila. "Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins." Investigative ophthalmology & visual science (2003)
Vander Jagt. "Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications." Journal of Biological Chemistry (1992)
Petchey, Michael. "Analysis of carbohydrates in lens, erythrocytes, and plasma by high-performance liquid chromatography of nitrobenzoate derivatives." Journal of Chromatography (1984)
@noordinary @lisaferraro @meatbag @Amazoniac @Such_Saturation @Koveras @ecstatichamster @haidut
"Before accepting a theory it is necessary not only to prove its own merit but also to explain why other theories are either wrong or not applicable." ―Paul Riordan Eva
I. Paul Riordan Eva
Much insight can be gained through the observations of Paul Riordan Eva, who critically examined the data regarding myopia. He had published an excellent review article in 1982, shown below:
Eva, Paul Riordan. "Refractive change in hyperglycaemia: hyperopia, not myopia." British Journal of Ophthalmology (1982)
In which he makes sense of the long‐noted observations of hyperglycemia on refractive changes—the propensity of the lens to bend light, and only real way to explain myopia. Trying to explain myopia through any other way leads to a contradiction: When imagining myopia to simply have as its cause the inability to focus, or a problem in controlling the iris, leads nowhere fast. That idea immediately falls apart when it's realized that anyone with nearsightedness can focus just fine, with glasses, and can easily bring objects far away into focus. The great majority of myopic changes are not a problem with the iris or any other muscle.
So a change in the refractive index has always been the primary focus among scientists, since there's no other logical way to explain it. The lens refractive index can actually change very quickly with raised blood glucose levels: Here are some quotes from Paul Riordan Eva to give you an idea of just how quick and drastic these changes can occur:
II. Case Reports—Excerpts
"Until that time she had had no visual symptoms since the onset of her diabetes in 1967, but within 3 hours of the first injection of 10 units of regular insulin she noticed blurring of vision in both eyes. She could not see with her glasses (OD −3·25), but she could see near without them. [...] An increase in the dosage of insulin led to gradual lowering of the blood sugar, followed later by a reversal of the refractive change. In January 1973 the refractive errors had returned to −3·25 in the right eye and −3·50 in the left with the same astigmatic corrections." ―Paul Riordan Eva
"In December 1980 he suddenly developed severe polydipsia and polyuria. On 15 December investigation by his internist showed a full blood sugar value of 350 mg/dl (19 mmol/l). Chlorpropamide, one tablet per day, was started. Although the patient discontinued this treatment within a few days because of side effects, frequent blood sugar estimations continued to show improvement. About 2 weeks after initiation of treatment the patient noticed a sudden change in his vision in that he could no longer see street signs while driving unless he took his glasses off. Examination by an optometrist on 8 January 1981 showed best visual acuity of 20/25 with corrections of −1·75 in each eye. Referral back to an ophthalmologist was advised. The patient's prediabetic refractive error was found to have returned on 27 February 1981. One other patient (patient 8) gave a similar history of suddenly being able to see in the distance without glasses, despite previously being myopic, about 2 weeks after starting oral medication for newly diagnosed diabetes." ―Paul Riordan Eva
III. History of the Observation
"The occurrence of transitory refractive changes in diabetic patients has been recognised since 1873." ―Paul Riordan Eva
These observations are in fact common, and have been noted for over a century. The diabetic cataract is certainly a well‐recognized pathology, and the diabetic refractive changes are recognized as well. Paul Riordan Eva documents observations along these lines dating back over a century:
"Duke-Elder in 1925 concluded that hyperopia is less common than myopia and that 'the refractive power of the eye tends to vary directly as the sugar content of the blood; that is, there is a tendency to hypermetropia with decreased sugar, with increased sugar to myopia." ―Paul Riordan Eva
The premise of Paul Riordan Eva's article appears to be a reminder to opthalmologists that hyperglycemia actually produces hyperopia, not myopia as sometimes thought. This is an important point to make note of, since this fact will be needed both to explain myopia itself and to suggest ways to improve it.
"More recently Duke Elder's rule has been refuted by Planten's study of 23 patients of whom only 2 became myopic with hyperglycaemia, the remainder all showing transient hyperopia." ―Paul Riordan Eva
"Every case showed transient hyperopia developing prior to the diagnosis of diabetes or soon after treatment was started," ―Paul Riordan Eva
The prevalence of myopic changes in hyperglycemia is fairly common, much more common than in non‐diabetic people. I think you'd be forced to agree that glucose plays a fundamental role in the refractive changes of the lens.
"Granstrom reported refractive change occurring as an initial symptom in 34% of diabetics and as an asymptomatic change in another 47%." ―Paul Riordan Eva
IV. The 'Explanation'
"Myopia with hyperglycaemia was then explained as being due to osmotic hydration of the lens due to salt retention." ―Paul Riordan Eva
Glucose became so well‐known to cause refractive changes off the lens that theoreticians felt obliged to explain it. Back then, in the early twentieth century, osmotic forces seemed the only logical way for doing so.
"Most authors accept Duke Elder's theory that refractive changes in diabetes are due to alterations in the power of the lens because of osmotic interactions between the lens and aqueous." ―Paul Riordan Eva
"...and provided an elaborate exposition of the possible role of osmotic forces, which established the concept without truly explaining the mechanism." ―Paul Riordan Eva
But there are flaws with such a theory. An influx of water caused by the hygroscopic forces of glucose would be expected to increase refraction, but the opposite is seen. What followed was a period of Rube Goldberg‐like ad hoc explanations of considerable magnitude.
"The resultant influx of water has been used to explain either a myopic or a hyperopic change, according to whether shape or refractive index is considered more important in determining overall refractive power." ―Paul Riordan Eva
And in fact, aldose reductase inhibitors have been shown in many studies to prevent these refractive changes in rabbits. It can no longer be argued that osmotic forces play a role in determining the width, shape, or refractive index of the lens itself. What is necessary to explain the effect of glucose on the lens necessarily lies downstream of aldose reductase, and none of those products are any more hygroscopic than glucose itself.
"Hyperglycæmia-induced refractive changes have been prevented in rabbits by aldose reductase inhibitors, although similar work in human subjects has yet to be published." ―Paul Riordan Eva
V. Aldose Reductase
This enzyme is an offshoot off normal glucose metabolism, and is only really active with the overflow of glucose seen in hyperglycemia. Under this enzyme, glucose is reduced to sorbitol. While not a dangerous molecule in itself, it doesn't have the protective phosphate group as it should. Too much glucose in the extracellular fluid—largely under the action of aldose reductase—leads to high amounts of trioses, acetol, and methylglyoxal: a common finding in diabetic blood.
"Methylglyoxal is also produced nonenzymatically during glucose metabolism." ―David L. Vander Jagt
What is unique about methylglyoxal, among all things, is its ability to form a covalent adduct with arginine. This forms a cyclic imidazalone ring, which is fluorescent. This species is often referred to as 'MG‐H,' with subscipts denoting subtle variations. Although sometimes called an 'adduct,' this is misleading as it implies simple addition—not the transformation which actually occurs. The end result is actually an imidazalone, a fluorescent ring.
 reaction process depicted; click to embiggen
reaction process depicted; click to embiggen(This ring is also sometimes called hydroimidazolone, but this isn't a very good name. The name that would make IUPAC the most happy would be Nᵟ‐(5‐hydro‐5‐methyl‐4‐imidazolon‐2‐yl)‐L‐ornithine. For short: it's better to accentuate the methyl group than the hydro and call it ornithyl‐methylimidazalone, or simply methylimidazalone. This name is used in publications found in the Journal of the American Chemical Society, so other people agree. The hydro is less conspicuous, and hence less deserving to be named. But of course, anyone can choose to name it after the much smaller hydrogen if they'd like. The point is: If anyone should decide to research this molecule, keep in mind that it goes by many names. It is even sometimes simply called an MG–arginine adduct. There's over six different names commonly used to describe this.)
Diabetic blood proteins are more fluorescent than normal people's for this reason, and taking L-arginine as a supplement has actually been used to counteract this. Such modifications are very well‐known among diabetes researchers.
"The glycation theory presents a quite different view of diabetic complications. In this theory, nonenzymatic modification of proteins by glucose, especially modification of longlived proteins such as lens crystalline or matrix proteins, is considered the critical event in the development of complications." ―David L. Vander Jagt
The lens is about 90% protein, and roughly 10% carbohydrate. The primary protein of the lens is called crystallin, for obvious reasons. This protein can certainly be covalently modified by methylglyoxal, and acetol to a lesser—but still significant—extent. David Vander Jagt had shown this in 1992.
 γ-crystallin from uniprot.org (click to embiggen)
γ-crystallin from uniprot.org (click to embiggen)The protein crystallin has many arginine residues, and thus would be expected to be covalently modified by methylglyoxal and acetol—products of the aldose reductase pathway.
"There are 10 to 20 arginine residues in the human crystallin isoforms. There is therefore an expectation that 10% to 20% of crystallin molecules have a MG-H modification." ―Naila Ahmed
VI. Proof of Imidazalone in the Lens
"Recently, we have found similar high concentrations of MG-H₁ in protein extracts of human blood cells and tissues of laboratory rats." ―Naila Ahmed
Though it may be fun to theorize about—analyze kinetic rates and blood glucose concentrations in attempt to estimate—we don't even have to. These covalent methylglyoxal and acetol adducts have been determined in the human lens in both hypoglycemic and euglycemic individuals.
Ahmed, Naila. "Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins." Investigative ophthalmology & visual science (2003)
It was found, predictably, that the diabetics had more methylimidazalone rings on the crystallin proteins—which constitute around 90% of the lens.
"We conclude that MG-H is a major AGE in human lens proteins quantitatively. The modification of lens crystallins by methylglyoxal led to a decrease in arginine residues and loss of positive charge." ―Naila Ahmed
"The formation of argpyrimidine is favored by high concentrations of methylglyoxal and oxidative processes." ―Naila Ahmed
At this point, things are becoming clear. The high glucose predictably leads to higher methylglyoxal and acetol concentrations, turning the arginine side‐chains of crystallin into fluorescent methylimidazalone side‐chains. Could this be expected to change the refractive index? I think so, and Naila Ahmed implies such a thing—giving us permission for thinking so (although we really don't need her permission).
"The increased glycation of proteins in cataractous lenses—particularly the higher extent of glycation by MG-H—may induce protein conformational changes that stimulate further glycation and oxidation and trigger protein aggregation leading to cataract." ―Naila Ahmed

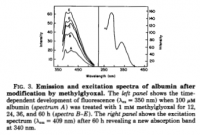
 click to embiggen
click to embiggenVII. The Molecular Refractive Index
"Furthermore, knowing the molecular refractive index contribution of macromolecular components is crucial for understanding the optical properties, structure, and function of different tissues in the eye." ―Huaying Zhao
Proteins have a refractive index, usually given as the 'increment.' This increment is a ratio of the refractive index over the concentration, given as dn/dc. The 'n' denotes the Snell refractive index, and the 'c' indicates concentration (why it has units of ml/g). The 'd' indicates the derivative, but I'll use the partial differential symbol '∂' to accentuate this. The reason an increment is given, and the reason it's a fraction with unexpected units, is that it depends on the concentration. When an aqueous protein solution is put in a tube with light shining through, the refraction of light depends also on concentration. Luckily however, the graph of refraction over concentration is constant, and what is arrived at is a constant number: the refractive index increment, or ∂n/∂c.


 click to embiggen
click to embiggenDifferent proteins have different refractive index increments, a value determined based only on the amino acids present. Since we're dealing with light, the amino acids tryptophan, tyrosine, and phenylalanine are of obvious significance.
"Clearly, there are considerable differences in the refractive properties of the amino acids, ranging from dn/ dc of 0.165 ml/g for proline to 0.277 ml/g for tryptophan. Amino acids with high polarizability and refractive index increment are those containing aromatic rings, sulfur, or double-bonds in the R-group, the highest ones being tryptophan, phenylalanine, tyrosine, histidine, cysteine, arginine, and methionine." ―Huaying Zhao
"We observed that the predicted dn/dc value correlates well with the fraction of residues being Arg, Asp, Cys, His, Met, Phe, Trp, or Tyr, which are those with the highest eight values. This correlation indicates that high dn/dc values are predominantly determined by the presence of these amino acids." ―Huaying Zhao
"We observed that the predicted dn/dc value correlates well with the fraction of residues being Arg, Asp, Cys, His, Met, Phe, Trp, or Tyr, which are those with the highest eight values. This correlation indicates that high dn/dc values are predominantly determined by the presence of these amino acids." ―Huaying Zhao
Relevant to the topic of myopia: the refractive index increment of crystallin—the protein of the eye—is found to be higher than any other protein. What would you expect? This protein was designed for this of course:
"Prominent examples of more extreme values include titin, with a predicted dn/dc 0.177 ml/g, and γ-crystallins with values in excess of 0.199 ml/g." ―Huaying Zhao
The types of amino acids present dictate the refractive index. Protein folding has surprisingly little effect. So powerful is this difference in only the amino acids themselves, they alone can actually reliably predict the refractive index increment of an entire protein.
"In the 1960s, McMeekin and colleagues determined the refractivities of amino acids, and proposed protein dn/dc values to be estimated from their amino acid composition. This approach compared very well with experimental protein dn/dc data." ―Huaying Zhao
So the osmolarity of glucose solution isn't the only way to change the refractive index of the lens, even if that explanation could ever be made to make sense. All that needs to be done—based on scientific measurements and described by Huaying Zhao's equations—is to change the relative proportions of the amino acids of the protein, with special emphasis given to the aromatic and fluorescent amino acids. To explain the observations of Paul Riordan Eva—that hypergylcemia is characterized by hyperopia—all one needs to explain is the change in one amino acid, or the transformation of arginine to fluorescent methylimidazalone: A covalent product formed from arginine and either methylglyoxal or acetol. This is the very same thing you'd expect from hyperglycemia—an increase in methylimidazalone—and the very same chemical species found by Naila Ahmed in human diabetic lens protein.
"In hyperopia, the image focuses behind the retina." ―Optics 101
An image focusing behind the retina would represent an increase in the refractive index increment (∂n/∂c) or the lens. The ∂n/∂c of tryptophan, tyrosine, phenylanine are the highest among the amino acids with values of 0.277, 0.240, and 0.244·ml/g, respectively (Zhao, Table 1). The ∂n/∂c of arginine is 0.206·ml/g, and is less than the aromatics. Since all aromatics—and histidine too—have a higher refractive index increment than those not cyclic, you'd expect methylimidazalone have a higher refractive index increment than arginine. For this reason, you would expect higher concentrations of methylglyoxal and acetol in the lens to increase the total refractive index and create hyperopia. This confirms all observations and leaves nothing wanting—with no paradox.
"The development of refractive abnormalities in response to hyperglycaemia is characterised by rapid onset followed by prolonged regression. In all our cases the hyperopic change occurred suddenly, never taking more than a few hours to appear. Return to the previous refractive state often required weeks, depending on how rapidly and reliably the blood glucose level was controlled." ―Paul Riordan Eva
The osmotic hypothesis cannot explain why hyperopia would form suddenly, and then take weeks to reverse. An osmotic theory would be depending on glucose, in real time, and also be at the whims of sodium concentration—never considered to be a factor in refractive changes. The sudden onset in the above case observed by Paul Eva can be explained by the transformation of arginine into methylimidazalone in the lens protein crystallin, thereby increasing the refractive index of said protein. The gradual recovery can be explained by the relatively‐slow protein turnover rate.
VIII. Myopia
"Previous investigators have generally believed refractive changes in diabetes, whether they be myopic or hyperopic, to be completely reversible." ―Paul Riordan Eva
Looking back on the analytical determinations of Ahmed, you can see that both the normal lens and the cataract lens have methylimidazalone. The diabetic lens has more, but only about twice as much as the controls—victims of 'injury or trauma.' It would then appear that the lens is normally modified by the products of methylglyoxal and acetol as a result of glucose metabolism: The lens naturally has some degree of methylimidazalone in place of arginine. There is no amino acid one‐letter code for this, and you could never see it denoted in the protein sequence anyway because it's a post‐translational modification—you could never really predict which arginine would be modified. Nonetheless, they exist. These fluorescent species with higher ∂n/∂c are found in the normal human lens (Ahmed, Table 1).
Hypoglycemia would be expected to cause myopia by attenuating the amount of methylimidazalone formed. This would lower refractive index increment back to that of arginine, and lower the global refractive index of the entire lens. This would cause the light to bend less, dropping short of the retina.
"In myopia, the image focuses before it gets to the retina." ―Optics 101
You would not expect myopia to form as quickly as hyperopia since the reversion back to arginine requires new protein synthesis, in the relative absence of glucose.
"There are 10 to 20 arginine residues in the human crystallin isoforms. There is therefore an expectation that 10% to 20% of crystallin molecules have a MG-H modification." ―Naila Ahmed
IX. Metabolic Considerations
The sorbitol pathway of the lens has been thoroughly‐studied: Enzyme's have been analyzed; they have been homogenized and centrifuged, then precipitated and further refined through column chromatography. Two glycolytic enzymes found in the lens have been kinetically measured under a variety of substrates. These two enzymes, aldose reductase and polyol dehydrogenase, are dependent on their cofactor NADH. This means that either a paucity of niacin or a change in redox balance could theoretically change methylglyoxal concentrations, and subsequent changes in lens refractive index. Also, if present, glutathione working through glyoxylase I & II would be expected to play a role. Glutathione would be expected to lower methylglyoxal concentrations through the well‐characterized glyoxal cycle (see Thornally).
"His idea of a decrease in the osmotic pressure of the aqueous in the face of hyperglycaemia, with consequent hydration of the cortical layers of the lens, has been reiterated by other authors. However, recent physiological evidence is to the contrary." ―Paul Riordan Eva


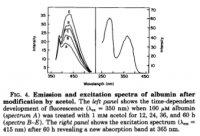
 click to embiggen
click to embiggenX. Wikipedia is Wrong
"Near-sightedness is due to the length of the eyeball being too long, far-sightedness the eyeball too short..." ―Wiki
Wikipedia is wrong, but that's only because I haven't yet gotten around to making the edit. Besides their wrongness in this particular case, they are also quite wrong about many other things.
XI. Miscellaneous Quotes
"Experimental data suggest that glucose diffuses into the lens and permeates the small extracellular space, which accounts for 5-10% of the lens volume," ―Paul Riordan Eva
"However, if glucose levels in the surrounding medium are increased, lens fibres seem to accumulate glucose [and produce methylglyoxal], and more especially-because of the apparent diversion of this substrate into the sorbitol pathway the metabolites sorbitol and fructose [and methylglyoxal and acetol]." ―Paul Riordan Eva
"The reason is probably saturation of the enzyme hexokinase, the starting point of the glycolytic and pentose phosphate pathways in combination with the markedly higher Km of aldose reductase, the first enzyme of the sorbitol pathway." ―Paul Riordan Eva
"The net result is an accumulation of fructose and sorbitol [and methylglyoxal and acetol downstream of that], both of which penetrate lens membranes poorly and are further metabolised to only a limited extent." ―Paul Riordan Eva
"Experiments with human lenses have shown that lens tissue is capable of forming sufficiently high levels of sorbitol [and methylglyoxal] with 24 hours of continuous hyperglycaemia," ―Paul Riordan Eva
"It would thus appear that a decrease in the refractive indices within the anterior portions of the lens might produce the hyperopia associated with hyperglycaemia." ―Paul Riordan Eva
"Recently, we have found similar high concentrations of MG-H₁ in protein extracts of human blood cells and tissues of laboratory rats." ―Naila Ahmed
"The formation of argpyrimidine is favored by high concentrations of methylglyoxal and oxidative processes." ―Naila Ahmed
"The activity of glyoxalase I and the concentration of triosephosphates are important variables controlling methylglyoxal concentration and related glycation in cultured rat lens." ―Naila Ahmed
"In this report, we found that MG-H was indeed the major methylglyoxal-derived AGE and that it was present at high concentration." ―Naila Ahmed
"The availability of accurate protein refractive indices is also crucial for understanding the biophysics of eyes. For example, dn/dc enters as a key parameter in models for light transmission and scattering in the cornea. Similarly, lens protein concentrations are often estimated from measured lens refractive indices on the basis of assumed crystallin dn/dc values." ―Huaying Zhao
"In a forthcoming communication, we show by systematic sequence analysis of crystallins of different members of the crystallin family that lens crystallins have indeed specifically evolved toward an elevated refractive index increment." ―Huaying Zhao
"As a consequence, the protein amino acid composition represents the major determinant for the protein refractive index increment, dn/dc." ―Huaying Zhao
"Physically, the refractive index of particles in the visible spectrum of light is a result of the local polarizability of the atoms and chemical groups due to deformation of the electron configuration about nuclei, and therefore insensitive to the long-range structure of macromolecules, and long known to be to a good approximation additive toward macromolecular refractivity." ―Huaying Zhao
"For a spherical lens, in which the refractive index distribution depends only on the distance from the lens centre, equations for the paths of rays through the lens can be derived from a knowledge of the refractive index distribution within the lens (Born and Wolf, 1970)." ―Melanie C.W.Campbell
XII. References
Bloemendal, Hans. "Lens protein." Critical Reviews in Biochemistry (1982)
Zhao, Huaying. "On the distribution of protein refractive index increments." Biophysical journal (2011)
Campbell, Melanie. "Measurement of refractive index in an intact crystalline lens." Vision Research (1984)
Eva, Paul Riordan. "Refractive change in hyperglycaemia: hyperopia, not myopia." British Journal of Ophthalmology (1982)
Jedziniak, Judith A. "The sorbitol pathway in the human lens: aldose reductase and polyol dehydrogenase." Invest Ophthalmol Vis Sci (1981)
Ahmed, Naila. "Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins." Investigative ophthalmology & visual science (2003)
Vander Jagt. "Reduction of trioses by NADPH-dependent aldo-keto reductases. Aldose reductase, methylglyoxal, and diabetic complications." Journal of Biological Chemistry (1992)
Petchey, Michael. "Analysis of carbohydrates in lens, erythrocytes, and plasma by high-performance liquid chromatography of nitrobenzoate derivatives." Journal of Chromatography (1984)
@noordinary @lisaferraro @meatbag @Amazoniac @Such_Saturation @Koveras @ecstatichamster @haidut
Last edited: