Nestito
Member
- Joined
- Dec 3, 2016
- Messages
- 60
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
Cannabinoids have been patented as anti inflammatory agents for decades.
Omega 3 have been known to have the same anti inflammatory properties.
But the difference is cannabinoids have been shown as well to kill cancer cells, especially in self treatment with Rick Simpson oil. In contrast, while there are some studies on omega 3 reducing tumor size and spread, the same dramatic effect hasn't been reported (to my knownledge at least).
This is a very interesting development.
Let's too remember flaxseed oil Is rich in both CBD (cancer cell killer) and omegas 3. It contains too linmarin, a form of B17 vitamin.
Mr Fahrenheit,I thought ω−3 fatty acids "worked" by blocking eicosanoid production. Only linoleic acid (ω−6) can be used to make eicosanoids.
Aspirin, colchicine, and indomethacin are also thought to reduce inflamation by inhibiting eicosanoid production.
Eicosanoids are powerful since they can bind to PPARγ, a nuclear receptor that—when activated—can direct the transcription of what I call "diabetes mode". An upregulation of proteins such as fatty acid synthesase and GLUT4 that orchestrate fat-storing activities in adipose tissue.
Although ω−3 fatty acids are "safe" from the perspective of eicosanoid production, they are still more prone to peroxidation that saturated fatty-acids. But from reading about lipofuscin, excessive iron and low antioxidant levels seem to be a bigger cause of intracellular peroxidation than unsaturated fats themselves.
Cold-pressed oils should have enough vitamin E and antioxidants to partially mitigate the susceptibility of unsaturated fatty acids to lipid peroxidation.
 Click to embiggen
Click to embiggen Click to embiggen
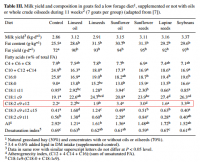
Click to embiggenThat's very helpful. Thanks!Well after reading quite a few animal studies on how only ω−6 linoleic acid causes cancer (eicosanoid pathway), I think the two most important ways of comparing fatty acid profiles are the iodine number and total linoleic acid content.
The iodine number is a decent estimation of peroxidation potential, and ties-in with free radicals and lipofuscin.
Linoleic acid is the only fatty acid that correlates very highly with cancer in animals, and the fully-saturated stearic acid is the only one consistently found protective.
The linoleic acid content of goat milk is low, and seems to vary little with diet.
View attachment 6188 Click to embiggen
From what I remember, the linoleic acid content of coconut is around 1–3%.
And the iodine value of butter is very low. Only coconut and palm out have lower iodine values.
View attachment 6189 Click to embiggen
The fatty-acid profile of milk is almost as Peatish as the coconut. The only strong criticisms of dairy products, that I am aware of, revolve around the hormone (both steroid and peptide) content and the potential of casein to form β-casomorphins.* The casomorphins aren't harmful physically, but can effect nociception and emotions in certain people.
There is also a tad more methionine in cow's milk than in coconuts (per gram protein), but less than the cow itself (beef). These can form polyamines which have interesting biological effects. I have only begun to read about polyamines.
*There is also the tie-in with vaccines, as casein is often used to culture the bacteria in which the vaccine's antigens derive from. Being injected with a protein can cause anaphylactic sensitization to that protein. For more information on this, see Charles Richet's Nobel Lecture and Vinu's articles on researchgate.
Do you know if coconut fat as good as dairy fat to protect you from dietary PUFA? Our Oregon instructor (not the one from New Hampshire) mentioned that one of the reasons why coconut oil keeps stable for a long time despite its PUFA content and lack of antioxidants is because the saturated fats don't allow destructive chains to propagate. However those fats are oxidized quickly once ingested, also they're not stored as easily as dairy fat and so I wonder if the protective effect only lasts while they are being oxidized. If possible, can you destroy my self-esteem on this?Well after reading quite a few animal studies on how only ω−6 linoleic acid causes cancer (eicosanoid pathway), I think the two most important ways of comparing fatty acid profiles are the iodine number and total linoleic acid content.
The iodine number is a decent estimation of peroxidation potential, and ties-in with free radicals and lipofuscin.
Linoleic acid is the only fatty acid that correlates very highly with cancer in animals, and the fully-saturated stearic acid is the only one consistently found protective.
The linoleic acid content of goat milk is low, and seems to vary little with diet.
View attachment 6188 Click to embiggen
From what I remember, the linoleic acid content of coconut is around 1–3%.
And the iodine value of butter is very low. Only coconut and palm out have lower iodine values.
View attachment 6189 Click to embiggen
The fatty-acid profile of milk is almost as Peatish as the coconut. The only strong criticisms of dairy products, that I am aware of, revolve around the hormone (both steroid and peptide) content and the potential of casein to form β-casomorphins.* The casomorphins aren't harmful physically, but can effect nociception and emotions in certain people.
There is also a tad more methionine in cow's milk than in coconuts (per gram protein), but less than the cow itself (beef). These can form polyamines which have interesting biological effects. I have only begun to read about polyamines.
*There is also the tie-in with vaccines, as casein is often used to culture the bacteria in which the vaccine's antigens derive from. Being injected with a protein can cause anaphylactic sensitization to that protein. For more information on this, see Charles Richet's Nobel Lecture and Vinu's articles on researchgate.
Yeah. There is also less chance of the first double-bond reacting with oxygen. I just read this passage in a book by Ilya Prigogine:Our Oregon instructor (not the one from New Hampshire) mentioned that one of the reasons why coconut oil keeps stable for a long time despite its PUFA content and lack of antioxidants is because the saturated fats don't allow destructive chains to propagate.
The rate of lipid peroxidation in bulk oils is usually modeled by the Arrhenius equation: ln(k) = ln(A) − E/RTFor the sake of example, let us take a simple reaction such as A + X = B + Y. This "reaction equation" means that whenever a molecule of component A encounters a molecule of X, there is a certain probability that a reaction will take place and a molecule of B and a molecule of Y will be produced. A collision producing such a change in the molecules involved is a "reactive collision." Only a usually very small fraction (for example, 10⁻⁶ of all collisions are of this kind. In most cases, the molecules retain their original nature and merely exchange energy.
Perhaps it could be fluidity as well? We all know that coconut oil is the least viscous, so it's molecules are moving around much faster than the other oils. You would expect more collisions with oxygen with coconut oil.Its remarkable stability may be caused by the presence of the endogenous antioxidants sesamol and sesaminol, together with tocopherols (24). Another contradictory result was found with the CtO [coconut oil]. The IV [iodine value] of CtO in the present study was almost half of that of PKO [palm kernal oil] (Table 1). However, the k [kinetic oxidation rate] value at a given T [temperature] for CtO was almost 60% faster compared to PKO. This may be caused by the initial high free fatty acid content in CtO. Unbound fatty acids are more prone to oxidation compared to fatty acids bound to the glycerol molecules. We postulate that this may be the main reason for the deviation from the norm for the k values in CtO.
This seems logical, but don't underestimate palmitic acid. This lipid can be found in cell membranes, and makes-up roughly ¹⁄₅ of the cell membranes in red blood cells in "average" people* from Pittsburgh, PA.However those fats are oxidized quickly once ingested, also they're not stored as easily as dairy fat and so I wonder if the protective effect only lasts while they are being oxidized.
@tyw,
I get the impression from reading your posts that you believe once you consume a diet that's consistently low in PUFA, the body will regulate them with less problems regardless of the ingestion of saturated fats as protection (previous posts here). This goes against the conception that it's the proportion of intake that matters. Can you massage my neurons with your opinion on this?
Why did you disappear?
Obligatory PUFA!My philosophy around PUFAs has always been to fulfil the obligate need for PUFA [!!!]...