Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
I had started this off to prove that the amount of membrane 'pumps' needed to maintain the membrane potential of the cell would exceed the surface area of such, mostly because @Waynish had suggested doing such a thing. However, according to my calculations there is enough membrane space to accommodate enough Na⁺/K⁺‐ATPase to maintain the membrane potential—assuming it does as advertised—using the value of permeability (P) that I had used. However, the values of permeability range considerably; and they actually vary enough that it could go either way. Using a different published value from the same article will give the result of not enough space—nine times not enough space.
There are still people who believe the protein currently known as Na⁺/K⁺‐ATPase does as advertised: specifically, it is generally believed to transport three Na⁺ ions across the cell membrane while importing two K⁺ ions per cycle. But biological 'pumps' are absurd on the face of it, and even more absurd once a person actually views the conformational change animations for this process. You don't have to go far to find criticisms of 'membrane pumps,' as the Gilbert Ling website is back up and full of arguments against Na⁺/K⁺‐ATPase mythology.
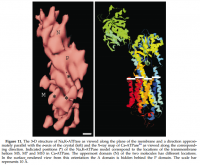
'The density map shows a protomer stabilized in the E₂ conformation which extends approximately 65‧Å × 75‧Å × 150‧Å in the asymmetric unit of the P2 type unit cell.' ―Hebert
The width of this enzyme can be known by looking at the structural data: High‐energy X‐rays are used to determine this because they have a relatively small 'wavelength' (spin) and a high energy; the longer 'wavelengths' on the EM spectrum disperse to readily upon interacting with matter and cannot provide good resolution for this reason.
Matching the length of 10‧Å scale bar with my screen‐capture program, replicating the resultant lines, and then pasting them across the transmembrane length of Na⁺/K⁺‐ATPase yields a value of 40‧Å (see below). This is a generous number, and can be considered the diameter of this more‐or‐less cylindrical protein.
 click to embiggen
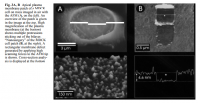
click to embiggenThe diameter of an MDCK cell is roughly 7.5‧μm, giving a surface area of 176.71‧μm²—an average value for for a cell. Now the only thing needed is the kinetic rate constant for Na⁺/K⁺‐ATPase, which are found just as easily. In fact, there are nine of them right here:
The cell membrane normally has a potential of around 60‧mV, and also a concentration gradient. This means that there will be a natural tendency to re‐equilibrate, to re‐establish a constant ionic concentration across the cell membrane. Indeed, this is what happens once the cell dies. The Goldman–Hodgkin–Katz flux equation allows one to calculate the flux of ions which will diffuse through the membrane in its resting state:
Φ = ΔC‧P‧z‧F‧ξ‧(exp(−ξ)/(1 − exp(−ξ))
ξ = z‧Ψ‧F/R‧T
ξ = z‧Ψ‧F/R‧T
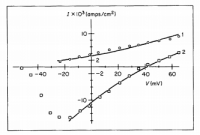
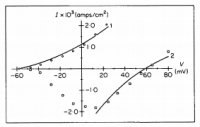
The membrane potential (Ψ) can be calculated with the Nernst equation for one ion species, or the Goldman–Hodgkin–Katz voltage equation (not to be confused with their flux equation) for multiple species. Using sample data in an online calculator dedicated for such a purpose, we get an a membrane potential of -67.92‧mV—an average value for such—using K⁺ ion concentrations of 150‧mM inside and 4‧mM outside; this gives us a difference in concentration across the membrane (ΔC) of 146‧mM. Now we have everything for the flux equation besides the membrane permeability (P), but this can be found in an '80s article on this equation; an article which had demonstrated how well it fits experimental evidence (see below):
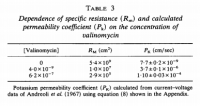 click to embiggen
click to embiggenSo with a permeability constant of 7.7×10⁻⁹‧cm/s all that is left to do is solve; all the other values are published constants:
ξ = z‧Ψ‧F/R‧T
ξ = 1‧(−67.92‧mV)‧(96,485‧J/V‧mol)/(8.314‧J/K‧mol)‧(310‧K)
ξ = (−6553.261)/(2577)
ξ = −2.54‧dimensionless
Φ = −(146‧mM) × (7.7×10⁻⁹‧cm/s) × (1) × (96,485‧J/V‧mol) × (2.54) × (ℯ²˙⁵⁴)/(1 − ℯ²˙⁵⁴)
Φ = −(2.755×10⁻⁷‧A/cm²) × (ℯ²˙⁵⁴)/(1 − ℯ²˙⁵⁴)
Φ = (2.755×10⁻⁷‧A/cm²) × (12.679)/(11.679)
Φ = 2.99×10⁻⁷‧A/cm²
ξ = 1‧(−67.92‧mV)‧(96,485‧J/V‧mol)/(8.314‧J/K‧mol)‧(310‧K)
ξ = (−6553.261)/(2577)
ξ = −2.54‧dimensionless
Φ = −(146‧mM) × (7.7×10⁻⁹‧cm/s) × (1) × (96,485‧J/V‧mol) × (2.54) × (ℯ²˙⁵⁴)/(1 − ℯ²˙⁵⁴)
Φ = −(2.755×10⁻⁷‧A/cm²) × (ℯ²˙⁵⁴)/(1 − ℯ²˙⁵⁴)
Φ = (2.755×10⁻⁷‧A/cm²) × (12.679)/(11.679)
Φ = 2.99×10⁻⁷‧A/cm²
And the units reduced to amps per distance squared, just as it should be. And since the cell surface area is 176.71‧μm², we then have for the total flux:
Φ = (2.99×10⁻⁷‧A/cm²) × (176.71‧μm²)
Φ = (2.99×10⁻⁷‧A/(10⁸‧μm²)) × (176.71‧μm²)
Φ = 5.2852×10⁻¹³‧A
Φ = (2.99×10⁻⁷‧A/(10⁸‧μm²)) × (176.71‧μm²)
Φ = 5.2852×10⁻¹³‧A
This is an ionic current, and amps can be converted into coulumbs per second—and then K⁺ ions per second. One coulomb is 6.242 × 10¹⁸ elementary charges, or that many K⁺ ions.
Φ = 5.2852×10⁻¹³‧C/s
Φ = 5.2852×10⁻¹³‧(6.242 × 10¹⁸‧K⁺)/s
Φ = 3.299×10⁶‧K⁺/s
Φ = three point three million potassium ions per second
Φ = 5.2852×10⁻¹³‧(6.242 × 10¹⁸‧K⁺)/s
Φ = 3.299×10⁶‧K⁺/s
Φ = three point three million potassium ions per second
Now lets take a look at Table 1 again: The Na⁺/K⁺‐ATPase with the fastest rate is 48.5‧K⁺/s each. So to balance the outward potassium flux with an equivalent outward flux, you'd need:
Φ = x‧(Na⁺/K⁺‐ATPase)
(3.299×10⁶‧K⁺/s)/(48.5‧K⁺/s) = x
(3.299×10⁶)/(48.5) = x
6.8×10⁴ = x
(3.299×10⁶‧K⁺/s)/(48.5‧K⁺/s) = x
(3.299×10⁶)/(48.5) = x
6.8×10⁴ = x
You'd need sixty eight thousand Na⁺/K⁺‐ATPase enzymes on the cell membrane. Having a diameter of ten angstroms gives each an area of 25π‧Ų (A = π‧r²). So many 'enzymes,' or membrane 'pumps.' on the cell would then occupy a total volume of:
25π‧Ų × (6.8×10⁴) = ?
170π‧Ų × 10⁴ = ?
170π‧(10⁻⁴‧μm)² × 10⁴ = ?
170π‧(10⁻⁴‧μm²)= ?
.0533‧μm²
170π‧Ų × 10⁴ = ?
170π‧(10⁻⁴‧μm)² × 10⁴ = ?
170π‧(10⁻⁴‧μm²)= ?
.0533‧μm²
Which is well within reason, occupying less than 1% of the available membrane space of 176.71‧μm². Hmm . . . so a person will have to prove this a different way, perhaps energetically. If I remember correctly: The terminal phosphate bond of ATP has an energy of ~7‧kJ/mol, which may not be enough energy to maintain the voltage gradient.. .
However, the values of permeability range by at least 10⁴‧fold. In one of Bowman's calculations (see text under Figure 1), he had used a value of 2.36×10⁻⁴‧cm/sec; this is ~3×10⁴ times larger that the one I'd used, making the final amount of required Na⁺/K⁺‐ATPase actually exceed the available space ninefold. So the membrane permeability values make a great deal of difference, something that probably underlies how mineralocorticoids work. Not many people know that the cell membrane has an experimentally‐confirmed aldosterone receptor (Wehling, 1992), and there's a good deal of evidence that Na⁺/K⁺‐ATPase is actually the membrane aldosterone receptor. Take for instance the two classic 'inhibitors' of this 'enzyme:' oubain and strophanthidin. These are steroidal molecules similar to aldosterone, and strophanthidin (left) has a striking similarity to spironolactone (right):
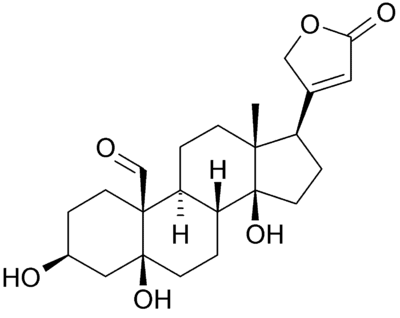
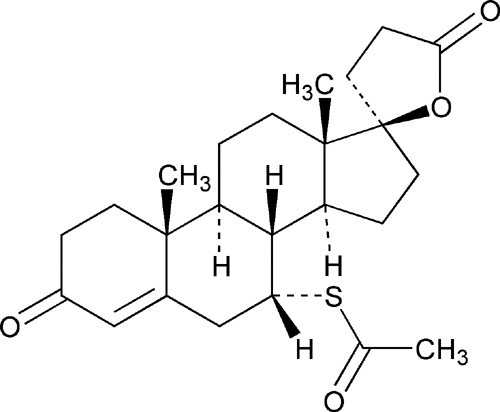
The way it appears to me is that Na⁺/K⁺‐ATPase is an ion pore under the natural influence of aldosterone and mineralocorticoids block this pore. This has been shown to occur in countless studies, and has the benefit of being realistic—no ridiculous 'pumping' action is required. The ionic gradient is simply created by the ~180‧mV mitochiondrial membrane potential within the cell, which in turn is created by the electrons stripped‐off of glucose (shuttled as hydride (∶H) on NADH, then flavin, and then coenzyme Q). This is balanced by the positive charges coming from the protons (H⁺) after the electrons are donated to the cytoskeleton, for nerve energy, and these protons are then used to transform ADP into ATP (Mitchell, 1967) which then diffuses to the cell membrane—repelled away from mitochondria by its substantial negative charge—releasing the H⁺ potential into the extracellular space. This is why urine is acidic and why membrane proteins hydrolyze ATP, simply to transport the worthless acidic protons (H⁺) out of the cell.
Hebert, Hans. "Three-dimensional structure of renal Na⁺/K⁺‐ATPase from cryo-electron microscopy of two-dimensional crystals." Journal of molecular biology (2001)
Lärmer, J. "Imaging excised apical plasma membrane patches of MDCK cells in physiological conditions with atomic force microscopy." Pflügers Archiv (1997)
Bowman, C. "Application of the Goldman-Hodgkin-Katz current equation to membrane current-voltage data." Journal of theoretical biology (1984)
Wehling, M. "Membrane receptors for aldosterone: a novel pathway for mineralocorticoid action." American Journal of Physiology-Endocrinology And Metabolism (1992)
Mitchell, P. "Chemiosmotic hypothesis of oxidative phosphorylation." Nature (1967)
Lärmer, J. "Imaging excised apical plasma membrane patches of MDCK cells in physiological conditions with atomic force microscopy." Pflügers Archiv (1997)
Bowman, C. "Application of the Goldman-Hodgkin-Katz current equation to membrane current-voltage data." Journal of theoretical biology (1984)
Wehling, M. "Membrane receptors for aldosterone: a novel pathway for mineralocorticoid action." American Journal of Physiology-Endocrinology And Metabolism (1992)
Mitchell, P. "Chemiosmotic hypothesis of oxidative phosphorylation." Nature (1967)
Last edited:
