Mega-Dose Thiamine: Beyond Addressing “Deficiency”
Throughout the past few years, I have been prescribing thiamine more and more often for individuals with a range of different health conditions. I have witnessed major symptomatic improvement in some people who displayed none of the key risk factors for thiamine deficiency, and many times had...
 www.eonutrition.co.uk
www.eonutrition.co.uk
Throughout the past few years, I have been prescribing thiamine more and more often for individuals with a range of different health conditions. I have witnessed major symptomatic improvement in some people who displayed none of the key risk factors for thiamine deficiency, and many times had been following clean, whole-food dietary regimes which contained levels beyond the RDA. The question I then began asking myself was this:
“Why does thiamine in sustained high doses work so well for such a wide variety of diseases? Is it merely addressing a deficiency, or is there something else going on here? ”
I have since come to the conclusion that one does not need to be deficient (in the nutritional sense) to benefit from this type of therapy, because high-dose thiamine is not simply working by correcting nutritional deficiency. Rather, thiamine is functioning as a metabolic stimulant to restore oxidative energy metabolism in cells that has been inhibited by factors unrelated to nutritional status.
Overwhelming toxicity and chronic oxidative stress have the capacity to inactivate thiamine-dependent enzymes involved in the generation of cellular energy, producing biochemical changes which are similar to clinical thiamine deficiency. This could basically be referred to as “functional” thiamine deficiency. In a functional deficiency dietary thiamine intake is somewhat irrelevant, because the concentrations obtained via the diet are simply not sufficient to overcome enzymatic inactivation.
Instead, extremely high concentrations of thiamine are often necessary to overcome the “metabolic block” and restore the deranged metabolism back to normal. Dr Derrick Lonsdale has discussed this concept on many occasions and laid out the theory in his various writings. In this article, I will explain the rationale behind high-dose thiamine therapy as a tool for bypassing these metabolic blocks and examine how this can be a useful therapy for chronic health conditions.
The basics of enzymes
To appreciate how thiamine’s potential utility in mega-doses, we should first look at the very basic function of enzymes. Enzymes are a type of protein that the body uses as a catalyst to facilitate or “speed up” the rate of biochemical reactions.Enzymes are responsible for driving the reactions involved in practically every known function of the human body, including building things up, breaking things down, modifying or changing molecules, and converting one molecule into another. Vitamins and minerals act as necessary cofactors or “helpers” for specific enzymes to work as they should.
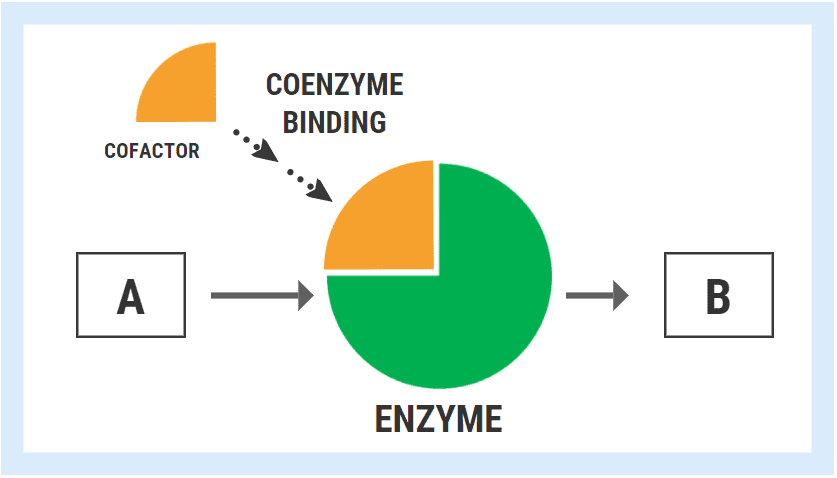
In the hypothetical diagram below, the enzyme responsible for converting substrate “A” into product “B” can only fulfill the task once it has bound its cofactor/coenzyme.
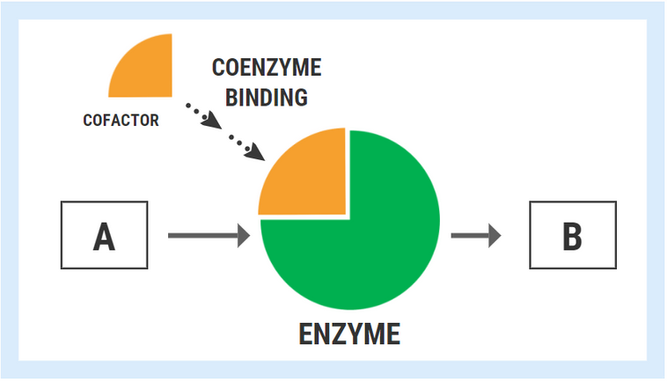
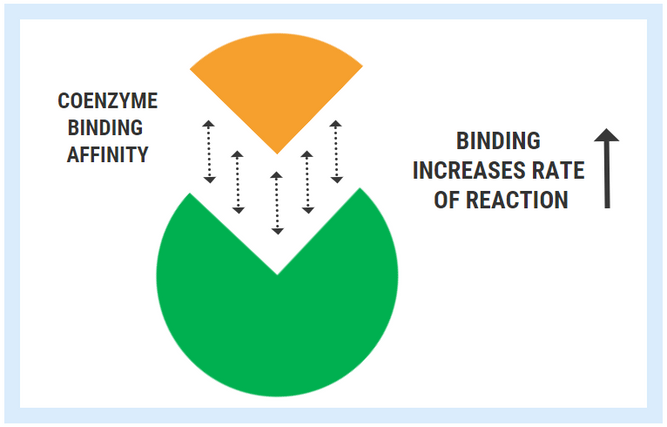
The ability of an enzyme to bind with its respective cofactor is referred to as the coenzyme affinity (km). A simple way to conceptualized this is to think of the enzyme like a magnet. Enzymes with high affinity for their coenzyme/cofactor exert a strong “magnetic” pull and can bind very readily with their coenzyme. With high coenzyme affinity and more binding, the activity of the enzyme speeds up and the rate of reaction (A->B) increases.
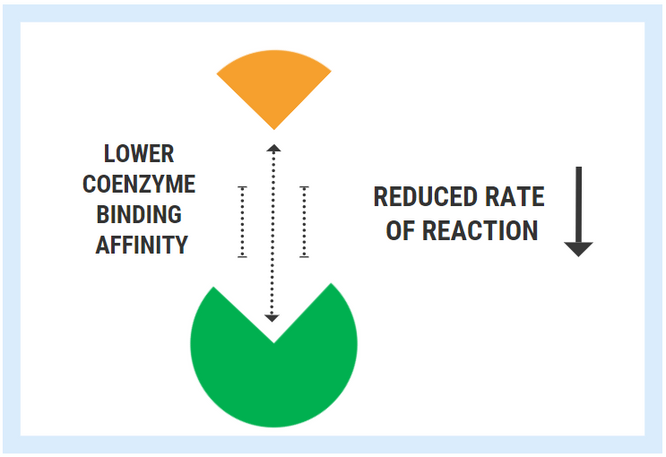
In contrast, enzymes with low coenzyme affinity exert a much weaker “magnetic” pull, meaning that they are less able to bind with the cofactor/coenzyme. Less cofactor binding means that the rate of reaction decreases.
A variety of inherited genetic conditions feature the production of defective enzymes with poor cofactor affinity. For these unfortunate individuals, the levels of nutrients found in food are simply not sufficient to overcome the genetically determined lack of affinity. However, a successful strategy used for these conditions is the administration of pharmacologic/mega-doses of the nutrient cofactor.
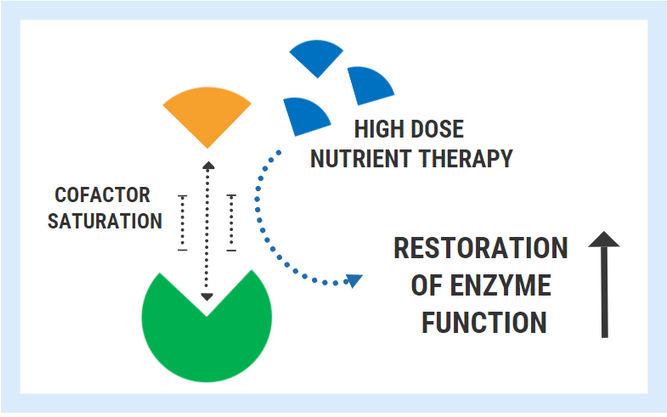
By saturating the cell, you can bypass the low affinity and restore enzyme function back to its normal state. Extremely high doses are often required to achieve this effect and this therapy must be maintained lifelong.
Examples of nutrient-responsive genetic conditions include:
- Thiamine-responsive maple syrup urine disease: A genetic defect in the branched chain ketoacid dehydrogenase enzyme results in remarkably low affinity for its coenzyme TPP. Continued high doses are necessary restore the function of this enzyme complex.
- Thiamine responsive Leigh’s disease: Inherited mutation in the gene encoding Pyruvate Dehydrogenase, with a decreased affinity for its TPP cofactor. Treated with pharmacological doses of thiamine to stimulate defective enzyme activity
- B12-responsive Methylmalonic acidaemia: Genetic defect encoding the methylmalonyl-CoA mutase enzyme, causing low affinity for adenosylcobalamin cofactor and a pathological accumulation of methylmalonic acid. This condition can be treated with megadoses of B12.
- Biotin-responsive holocarboxylase synthetase deficiency: Genetic mutation renders biotin-responsive carboxylase enzymes much less able to bind with biotin cofactor due to markedly decreased affinity. Supraphysiologic doses can restore normal enzyme function
- B6-responsive homocysteinuria: A rare defect in the cystathionine-B-synthase enzyme reduces affinity for its coenzyme pyridoxal-5-phosphate. This leads to the toxic buildup of homocysteine. Mega-doses of vitamin B6 can return enzyme activity back to normal.
Not just genetics
The activity of different enzymes is tightly regulated depending on metabolic requirements, energy intake, and numerous other conditions within the cell. In simplified terms, if cells need to break something down, build something up, slow a process down, speed a process up (etc), the activity of the enzymes involved in those pathways will reflect that. Enzyme activation/inhibition is a necessary part of normal cell physiology.
However, the activity of specific enzymes can also be affected by other factors including toxins. There are certain enzymes involved in energy metabolism which are particularly susceptible to inactivation by free radicals and oxidative damage. Short-term, this is most likely beneficial. But under conditions of chronic oxidative stress, such as that found in chronic disease, enzyme inactivation can become pathological.
A key enzyme involved in mitochondrial energy metabolism called alpha ketoglutarate dehydrogenase (KGDH). Several nutrients serve as cofactors for this enzyme complex, with thiamine taking center stage. KGDH is a rate-limiting step in in the TCA cycle, meaning that when this enzyme slows down, every other downstream step also slows down. Whilst a deficiency of any of the necessary cofactors will reduce the activity of this enzyme, it is also exquisitely sensitive to oxidative stress. KGDH appears to be more sensitive to disturbed homeostatic factors than other enzymes, playing the role of a metabolic redox sensor, capable of switching oxidative phosphorylation “on” or “off” depending on the cellular redox state and requirement for energy. Reactive oxygen species will selectively inactivate the KGDH complex and slow down oxidative energy metabolism. This inhibition is functionally beneficial for cells in the short-term as an attempt to avoid energy overload and oxidation. Not only is KGDH a target of oxidative inactivation, but it is also a significant generator of oxidative free radicals. Here, it plays a regulatory role which clearly serves essential functions in maintaining cell homeostasis.
However, under long-term conditions of oxidative stress, chronic KGDH inhibition is thought to be a driving factor underlying many neurodegenerative diseases. In chronic fatigue syndrome, recent metabolomic analysis found that one of the few metabolites (out of 800+) elevated with statistical significance was alpha-ketoglutarate, which is perhaps also consistent with chronic KGDH inhibition. Several toxic and inflammatory factors have also been shown to inhibit KGDH. Immune cells in the brain called microglial are involved in neuroinflammation and can be activated by a variety of stressors including toxins, trauma, and infectious insult (think Lyme, or LPS coming from a leaky BBB). Microglia produce myeloperoxidase and downstream products including hypochlorous acid and mono‐N‐chloramine – all of which are powerful inhibitors of KGDH. Heavy metals including aluminium and arsenic, along with fungal mycotoxins inhibit thiamine-dependent enzymes including KGDH and pyruvate dehydrogenase (PDHC).
Activated microglia caused by inflammation in the brain generate excess amounts of nitric oxide and its free radical peroxynitrite, both of which further inactivate KGDH. Polyunsaturated fats lining neuronal membranes are prime targets for oxidative damage in the brain, yielding a toxic byproduct called hydroxynonenal (HNE). Once more, HNE was shown to inactivate both KGDH and PDHC, whereas other mitochondrial enzymes were unaffected.
Endogenous neurotoxins such as MPP+ and isoquinolone derivatives (breakdown products of catecholamine neurotransmitters) have been associated with Parkinson’s disease, and also inactivate KGDH. These metabolites include oxidized derivatives of dopamine and norepinephrine. Other KDHC and PDHC inhibitors include the breakdown products of halogenated toxic chemicals such as Tetrafluoroethylene (TFEC).
Oxidative stress and chronic inflammation are the hallmarks of chronic disease – whether it be due to chronic infection, toxicity, biotoxin exposure, or whatever else - and both factors appear to inhibit/inactivate KGDH.
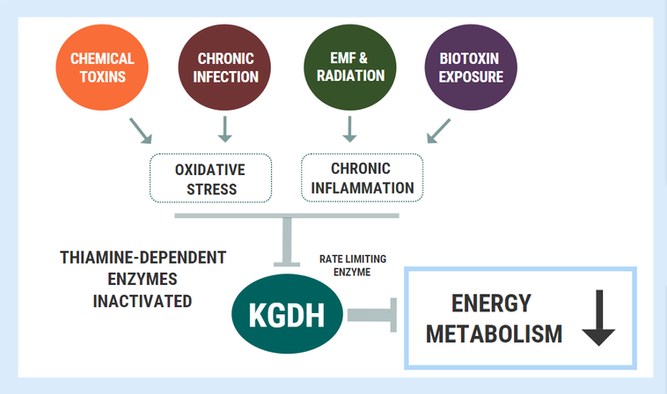
As the rate-limiting step in oxidative phosphorylation, the chronic inhibition of this enzyme can spell devastating consequences for cellular energy turnover. A person could be obtaining a great amount of thiamine through their diet, but the underlying inhibition of these enzymes will produce the exact same outcomes as a dietary “deficiency”. In other words, these changes will induce a functional deficiency.
Mega-dose thiamine to the rescue
When enzyme inhibition becomes pathological, we can apply similar principles as outlined above with nutrient-responsive genetic conditions. We can use high doses to bypass or overcome the metabolic blocks caused by enzyme inhibition through saturating the enzymes with ultra high doses. This concept was wonderfully illustrated in a study titled: “Thiamine preserves mitochondrial function in a rat model of traumatic brain injury, preventing inactivation of the 2-oxoglutarate dehydrogenase complex”.
In this study on several groups of rats who were not deficient in thiamine, researchers investigated the effects of traumatic brain injury (TBI) on brain energy metabolism. They showed that the oxidative stress associated with TBI inactivated the KGDH enzyme, causing great reductions in energy synthesis which was coupled with brain damage.
However, administering massive doses of thiamine to the rats before TBI was able to completely protect the KGDH enzyme. The thiamine-treated group maintaining normal activity of KGDH, mitochondrial respiration, and ATP despite being exposed to the injury. Furthermore, the restoration/protection of KGDH might have also conferred some degree of cytoprotection by combating inflammation, which was demonstrated by reduced inflammatory gene expression at 3 days post-TBI.
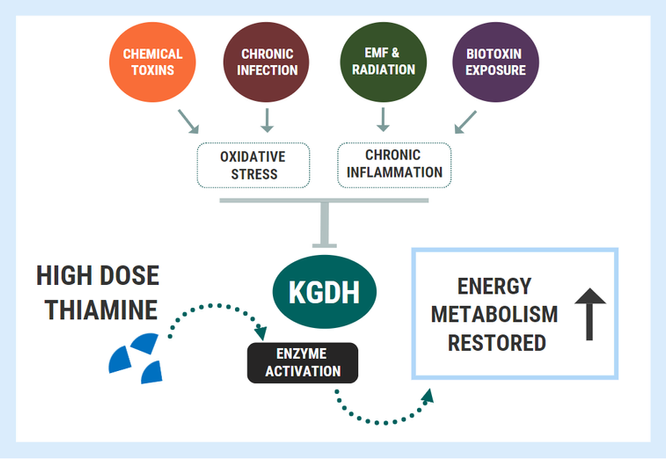
This demonstrated was that very high doses of the cofactor could provide protection against an insult which was not related to deficiency! In fact, similar results have been shown in several other studies:
Thiamine administration protected neurons against inflammation-induced impairments in neurogenesis caused by exposure to radiation, both in vitro and in vivo. Thiamine treatment also significantly increased lifespan. Attenuation of these inflammatory effects are thought to be due to increased stimulation of KGDH activity.
A more recent study also looked at traumatic brain injury (TBI) with a focus on glutamate neuroexcitotoxicity. They showed that excess nitric oxide and peroxynitrite found in neuroinflammation led to the inactivation of KGDH. KGDH inhibition reduced glutamate uptake into the Kreb’s cycle, producing glutamate excitotoxicity and neuronal cell death. Once again, extra levels of thiamine reversed this issue by stimulating KGDH, increasing glutamate clearance and protecting the cells against injury.
The authors concluded:
“Thus, the impairment of OGDHC [KGDH] plays a key role in the glutamate mediated neurotoxicity in neurons during TBI; pharmacological activation of OGDHC may thus be of neuroprotective potential. "
Interesting choice of words, huh? They are basically telling us that the pharmacological use of thiamine might be helpful in conditions where KGDH is inactivated, and enzymatic stimulation can be protective against glutamate neuroexcitoxicity.
For the readers reference, here are a quick list of conditions which are thought to involve neuroexcitotoxicity as part of the disease-process:
- ME/Chronic Fatigue Syndrome
- Neurodegenerative diseases : ALS, Huntingtons & Alzheimer’s
- Depression, Autism and Schizophrenia
- Parkinson’s and MS
- Electromagnetic hypersensitivity syndrome
- Multiple chemical sensitivity
- Neuroborelliosis (Lyme)
However, this is likely also related to the stimulation of transketolase activity (a thiamine-dependent enzyme involved in replenishing reduced glutathione). Under conditions of oxidative burden and increased requirement for glutathione recycling, there is a need for increased transketolase activity and thiamine.
High doses thiamine will stimulate the transketolase enzyme to maintain glutathione levels. This was shown in a different study using metabolomic analysis in cardiac ischemia, which found increased levels of ribulose-5-phosphate suggestive of increase TKT activity. Indeed, both thiamine and benfotiamine were found to increase the genetic expression and activity of the transketolase enzyme to counteractive oxidative damage and cell injury in diabetic vascular endothelial dysfunction.
High doses of thiamine can also restore activity of the pyruvate dehydrogenase enzyme complex in the face of inactivation. Cardiac arrest was shown to markedly depress PDHC activity through inactivation. In rats, high-dose thiamine post-cardiac arrest restored pyruvate dehydrogenase activity in brain, mitochondrial respiration, improved neurological function, reduced brain injury, and improved survival at 10 days. The quantity of the enzyme did not change, showing that thiamine worked by stimulating PDHC activity at high doses, thereby preventing injury-induced inactivation of this enzyme complex. Pre-treatment with thiamine pyrophosphate protected against cardiac ischemia by maintaining mitochondrial function, ATP concentrations, and inhibiting mitochondrial fission
Furthermore, copper toxicity was shown to inactivate the PDHC , produce mitochondrial dysfunction and neurological damage in rats. High doses of thiamine protected against the inhibition of Pyruvate dehydrogenase, markedly extended life span and protected against neuronal death.
Human evidence
The late Italian neurological A. Constantini published several case studies on the use of mega doses of thiamine for different conditions and saw impressive results.
In one of the case reports on fibromyalgia, two patients saw an abrupt and immediate improvement only when they reached 1,800mg per day. At lower doses, improvements were negligible.
High dose thiamine produced appreciable improvements in fatigue in 15 MS patients. Likewise, high doses were shown to produce remarkable and rapid improvement in the neurological condition essential tremor. Severe chronic fatigue in IBD patients with normal thiamine lab tests was reversed in most patients with megadoses.
Thiamine injections completely reversed gait abnormalities and motor failure in two patients with Freidrick’s Ataxia.
Importantly, Constantini and colleagues concluded:
“From this clinical observation, it is reasonable to infer that a thiamine deficiency due to enzymatic abnormalities could cause a selective neuronal damage in the centers that are typically affected by this disease.”
Furthermore, in a case report of two patients, dystonia was reverse with thiamine administration. I have also seen this occur in several children with autism and/or neurodevelopmental abnormalities. Another case report detailed high-dose thiamine injection in patients with Parkinson’s disease, all of which had “normal”plasma thiamine levels (meaning that they were not classically diagnosed as having deficiency). The patients experienced between 30 and 77% improvement in motor coordination. We have seen from the research above that the neurotoxic metabolites which are thought to drive Parkinson’s also have the strong capacity to inhibit thiamine-dependent enzymes. It is therefore no wonder why thiamine can have such as tremendous impact on this condition.
Him and his colleagues completed a larger study on 50 patients two years later, and found that 100mg thiamine injection twice per week produced massive improvement in both motor and non-motor symptoms, with some patients experiencing complete clinical remission.
Are these results simply addressing a deficiency, or is something else going on here?
The daily recommended dietary intake of thiamine is merely 1 – 1.5 mg per day. Surely, if the benefits were simply due to nutritional repletion then we would see benefits at similar levels, or even 10x that amount? Except we do not. Rather, most people are required to consume one hundred to one thousand times the daily recommended intake to see restoration of metabolism and symptomatic improvement. This is what I see in clinical practice on a frequent basis, and this is also what has been demonstrated in the case literature.
The sheer amount of the nutrient necessary for clinical improvement is not consistent with simply addressing a deficit. Nutritional repletion is by no means an adequate explanation for this magnitude of effect. It IS consistent with stimulating enzyme activity to overcome inactivation, however.
Constantini hit the nail on the head with one quote from another paper:
“ We may suppose that symptoms decrease when the energetic metabolism and other thiamine-dependent processes return to physiologic levels. Our aim was not to correct a systemic deficit of thiamine, but rather to increase the activity of enzymes involved in cell production energy in selective brain regions;
Indeed, Constantini understood that thiamine could be used as metabolic enhancement to stimulate the enzymes involved in energy metabolism which had otherwise been inhibited by other factors. This is where we are dealing with a “functional deficiency” which can only be addressed by supraphysiologic concentrations to saturate the cell for improved bioenergetics. As I said mentioned previously, Dr Derrick Lonsdale has highlighted on many occasions how thiamine’s effective is due to its pharmacological action, rather than nutritional repletion.
Rather than remaining hyperfocused on correcting a deficit, we can be using this molecule to improve bioenergetics regardless of nutrient status. This means that someone does not necessarily need to be nutritionally deficient to benefit from thiamine supplementation at high doses.
It is worth noting here that there are a few other variables which I have not discussed thus far. Outside of the context of genuine inherited genetic defects, there are numerous polymorphisms in genes related to thiamine transport and metabolism. These polymorphisms can influence enzyme activity, albeit to a lesser extent, and can predispose one to developing a deficiency. Nonetheless, this does not alter the fundamental principles laid out in this article.
It is also important to understand that the clinical improvements demonstrated are not just due to thiamine’s role as a cofactor to drive biochemical reactions to their completion.
Rather, this nutrient exerts numerous non-coenzyme functions including allosteric regulation of other enzymes in energy metabolism, direct anti-oxidant and anti-inflammatory actions. It has been shown to influence the transcription of genes involved in modulating and dampening inflammation and oxidative stress upstream.
Thiamine and benfotiamine supplements exhibit “anti-stress” properties in the brain, protecting against stress induced suppression of hippocampal neurogenesis. These effects stem from anti-oxidant, rather than coenzyme roles.
Here are a list of some of the non-coenzyme targets of high dose Benfotiamine:

Some other non-cofactor roles include:
- Thiamine pyrophosphate protected rat liver against cisplatin toxicity, whilst thiamine was just as effective as NAC in protecting the liver against acetaminophen toxicity
- Thiamine was shown to protect cells against radiation induced genetic changes and injury
- Thiamine and benfotiamine both counteract ultrasound-induced aggression, normalize AMPA receptor expression and plasticity markers, and reduce oxidative stress in mice
- High dose thiamine alleviates biomarkers related to oxidative stress and inflammation in lead toxicity in animal research
- Thiamine pyrophosphate prevented infertility induced by ischemia-related injury in rats
- Pretreatment with high doses of thiamine also protected cardiac myocytes against hypoxia-induced apoptosis and DNA fragmentation through increasing Heat-shock-protein 70.
- Thiamine improves markers of metabolic dysfunction and glucose metabolism and exerts anti-fatigue effects by attenuating the decrease in ATP content in skeletal muscle fatigued induced by workload.

