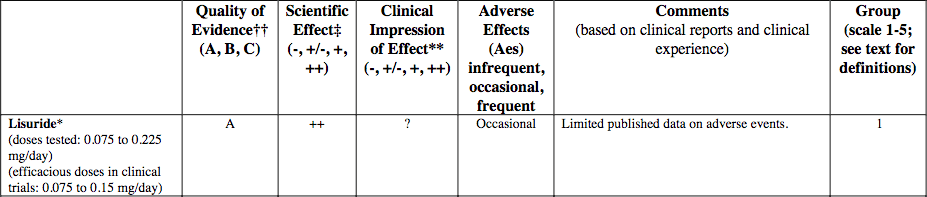
After many months of often fruitless searching and negotiations with chemical vendors from all over the world, I am pleased to finally announce the release of our Lisuride product. This is perhaps the most anticipated product we have ever released and it is for a good reason. The recent acquisition of terguride (a lisuride isomer) by Pfizer with the intent of treating heart, liver, lung, and other fibrosis demonstrates the commitment of Big Pharma to the correct idea that serotonin is a causative factor in the pathology of so many conditions. It is not a "happy hormone" at all, unless you consider a zombified, fibrotic state to be a form of bliss. Lisuride is one of the most potent dopamine agonists currently in clinical or research use. It is active across most known dopamine receptors (D1, D2, D3, D4, and D5). In addition, it is an antagonist on several serotonin receptors such as 5-HT2B, 5-HT6, and 5-HT7. The systemic effects of lisuride have been described in studies as both dopaminergic and anti-serotonergic. It is the antagonism of 5-HT2B that makes lisuride and its cousin terguride interesting for various fibrotic conditions. It is also worth noting, that lisuride is one of the few known so-called "inactivating" antagonists on the serotonin receptors 5-HT6 and 5-HT7.
5-HT7 receptor - Wikipedia, the free encyclopedia
An inactivaing antagonist renders a receptor persistently unable to react to an agonist such as serotonin or a serotonergic drug. This is similar to the effects of "suicidal" aromatase inhibitors like exemestane, which permanently deactivate the aromatase enzyme. These properties of lisuride suggest that it can have a very long-term serotonin antagonistic effects on specific receptors, and even a single dose can manifest its effects long after the lisuride is excreted from the body. This makes lisuride able to achieve beneficial effects even with very infrequent dosing, which has been confirmed in clinical trials at least in regards to lowering prolactin.
Lisuride - Wikipedia, the free encyclopedia
"...Lisuride is used to lower prolactin and, in low doses, to prevent migraine attacks. The use of lisuride as initial anti-Parkinsonian treatment has been advocated, delaying the need for levodopa until lisuride becomes insufficient for controlling the parkinsonian disability. Preliminary trials suggest the dermal application of lisuride may be useful in the treatment of Parkinson's disease. As lisuride is very poorly absorbed when take orally and has a short half-life, continuous transdermal administration offers significant advantages and could make the compound a far more consistent therapeutic. Lisuride is not currently available in the US, as the drug was not a commercial success in comparison with other dopamine receptor agonist antiparkinsonian compounds."
The units listed on the label are just for measurement purposes. They do not indicate suggested or optimal dose. Please note that similar to the products sold by companies like BlueSky, this product if for lab/research use only. The product can be ordered from the link below:
IdeaLabs Online Store - Worldwide Ordering And Delivery - Laboratory Research Chemicals
*******************************************************************************
Lisuride is a chemical agent of the iso-ergoline class, related to the dopaminergic ergoline Parkinson's drugs. Lisuride is a full dopamine and a mixed agonist/antagonist for several serotonin receptors. It is an antagonist at the serotonin 5-HT2B receptor, which is its main mechanism of action in reversing fibrosis of various tissues and organs. It has a high affinity for the dopamine D2, D3 and D4 receptors, as well as D1, and D5. It is an agonist of 5-HT1Aand 5-HT2C receptors, and putative antagonist on 5-HT2A (which explains its antagonism to LSD hallucinogenic effects). Lisuride is also a putative histamine antagonist, on both H1 and H2 receptors.
Drops per container: about 240
Each drop contains the following ingredients:
Lisuride (maleate): 25 mcg
Other ingredients: add product to shopping cart to see info
Note: Liquid preparations of lisuride are best kept at a temp of 20 degrees C or lower to ensure shelf-life of at least 3 months.
*******************************************************************************
References:
Miscellaneous
Influence of lisuride, a dopaminergic agonist, on the sexual function of male patients with chronic renal failure. - PubMed - NCBI
"...The increase of plasma testosterone was greater in hyperprolactinemic patients (86% v 15% in normoprolactinemic) and was accompanied by a clear improvement in the studied parameters of sexual behaviors. The response of PRL to TRH was modified in hyperprolactinemic patients while that of LH and FSH to LH-RH was not modified, although Lisuride induced an increase of the basal value of LH (P less than 0.01) in the hyperprolactinemic group. The drug was fairly well tolerated, did not induce hypotension, and the overall incidence of side effects decreased along the study."
Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-Hydroxytryptamine7 receptor by risperidone, 9-OH-Risperidone, ... - PubMed - NCBI
Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. - PubMed - NCBI
Pharmacological properties of a wide array of ergolines at functional alpha(1)-adrenoceptor subtypes. - PubMed - NCBI
In vitro pharmacology of clinically used central nervous system-active drugs as inverse H(1) receptor agonists. - PubMed - NCBI
Glutamate-induced cell death and formation of radicals can be reduced by lisuride in mesencephalic primary cell culture. - PubMed - NCBI
Mastalgia: a review of management. - PubMed - NCBI
Involvement of 5-hydroxytryptamine (HT)7 receptors in the 5-HT excitatory effects on the rat urinary bladder. - PubMed - NCBI
No evidence of the usefulness of eye blinking as a marker for central dopaminergic activity. - PubMed - NCBI
Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine1A receptor-mediated behavioral effects overlap its other properties in rats. - PubMed - NCBI
Lisuride acts at multiple sites to induce ocular hypotension and mydriasis. - PubMed - NCBI
Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. - PubMed - NCBI
Specific involvement of central 5-HT1A receptors in the mediation of male rat ejaculatory behavior. - PubMed - NCBI
The effects of lisuride, terguride and bromocriptine on intraocular pressure (IOP). - PubMed - NCBI
Comparative effects of LSD and lisuride: clues to specific hallucinogenic drug actions. - PubMed - NCBI
Antagonism of L-5-hydroxytryptophan-induced head twitching in rats by lisuride: a mixed 5-hydroxytryptamine agonist-antagonist? - PubMed - NCBI
Influence of lisuride, a dopaminergic agonist, on the sexual function of male patients with chronic renal failure. - PubMed - NCBI
Serotonin involvement in lisuride-induced mounting and in sleep. - PubMed - NCBI
Stimulation of dopamine autoreceptors elicits "premature ejaculation" in rats. - PubMed - NCBI
Lisuride, LY-141865, and 8-OH-DPAT facilitate male rat sexual behavior via a non-dopaminergic mechanism. - PubMed - NCBI
Spinal control of sexual behavior: effects of intrathecal administration of lisuride. - PubMed - NCBI
Spinal control of sexual behavior: Effects of intrathecal administration of lisuride - ScienceDirect
Lysergic acid diethylamide (LSD) and lisuride: differentiation of their neuropharmacological actions. - PubMed - NCBI
Serotonergic and dopaminergic effects of yawning in the cat. - PubMed - NCBI
The interaction of lisuride, an ergot derivative, with serotonergic and dopaminergic receptors in rabbit brain. - PubMed - NCBI
Inhibition of neurotransmitter receptor binding by ergot derivatives. - PubMed - NCBI
Stimulating effects of lisuride on masculine sexual behavior of rats. - PubMed - NCBI
Induction of mounting behaviour in female and male rats by lisuride. - PubMed - NCBI
Neurological/Brain
Lisuride reduces involuntary periodic leg movements in spinocerebellar ataxia type 2 patients. - PubMed - NCBI
Drug Treatments for the Prevention of Migraine Headache - PubMed - NCBI
Anticonvulsive effects of the dopamine agonist lisuride maleate after experimental traumatic brain injury. - PubMed - NCBI
Lisuride treatment of restless legs syndrome: first studies with monotherapy in de novo patients and in combination with levodopa in advanced disease. - PubMed - NCBI
Transdermal lisuride: short-term efficacy and tolerability study in patients with severe restless legs syndrome. - PubMed - NCBI
Transdermal lisuride delivery in the treatment of Parkinson's disease. - PubMed - NCBI
The dopamine receptor agonist lisuride attenuates iron-mediated dopaminergic neurodegeneration. - PubMed - NCBI
Protection of dopaminergic neurons in primary culture by lisuride. - PubMed - NCBI
Lisuride treatment of Alzheimer's disease. A preliminary placebo-controlled clinical trial of safety and therapeutic efficacy. - PubMed - NCBI
Lisuride prevents learning and memory impairment and attenuates the increase in extracellular dopamine induced by transient global cerebral ischemi... - PubMed - NCBI
Neuroprotection by dopamine agonists. - PubMed - NCBI
[Effect of lisuride on experimental cerebral infarction in rats]. - PubMed - NCBI
Antagonist effect of terguride in Parkinson's disease. - PubMed - NCBI
Chronic lisuride hydrogen maleate administration enhances muscarinic receptor binding in senescent rat brain. - PubMed - NCBI
Intravenous administration of lisuride in the treatment of neuroleptic malignant syndrome. - PubMed - NCBI
[Lisuride for the prevention of migraine. Results of a multicenter study]. - PubMed - NCBI
Effects of lisuride hydrogen maleate on ischemia-induced depletion of brain acetylcholine levels in gerbils. - PubMed - NCBI
Low dose lisuride in advanced Parkinson disease. - PubMed - NCBI
[Analysis of the clinical experience with 136 patients with headache treated with lisuride]. - PubMed - NCBI
Acute treatment of Huntington's chorea with lisuride. - PubMed - NCBI
Beneficial effects of lisuride in Meige disease. - PubMed - NCBI
The use of lisuride, a potent dopamine and serotonin agonist, in the treatment of progressive supranuclear palsy. - PubMed - NCBI
Dopamine agonists and cobalt-induced epilepsy in the rat. - PubMed - NCBI
Lisuride, a potent drug in the treatment of muscular rigisity in rats. - PubMed - NCBI
Mood/Cognition/Behavior/Addiction
[Treatment of premenstrual tension syndrome (PMS) with lisuride maleate]. - PubMed - NCBI
The dopamine agonists lisuride and piribedil protect against behavioural and histological changes following 4-vessel occlusion in the rat. - PubMed - NCBI
Striatal dopamine receptors and adenylyl cyclase activity in a rat model of alcohol addiction: effects of ethanol and lisuride treatment. - PubMed - NCBI
The effects of lisuride on mood and sleep during acute withdrawal in stimulant abusers: a preliminary report. - PubMed - NCBI
Lisuride reduces intravenous cocaine self-administration in rats. - PubMed - NCBI
[Therapeutic effect of lisuride maleate on post-stroke depression]. - PubMed - NCBI
Lisuride reduces psychomotor retardation during withdrawal from chronic intravenous amphetamine self-administration in rats. - PubMed - NCBI
Treating organic abulia with bromocriptine and lisuride: four case studies. - PubMed - NCBI
[Anxiolytic effects of lisuride and its agonistic action to central 5-HT1A receptors]. - PubMed - NCBI
[Effects in animal models of depression of lisuride alone and upon coadministration with antidepressants]. - PubMed - NCBI
Recovery of memory following forgetting induced by depletion of biogenic amines. - PubMed - NCBI
CVD/Circulation
Fibrotic heart-valve reactions to dopamine-agonist treatment in Parkinson's disease. - PubMed - NCBI
Lisuride, a dopamine receptor agonist with 5-HT2B receptor antagonist properties: absence of cardiac valvulopathy adverse drug reaction reports sup... - PubMed - NCBI
Potential vascular alpha1-adrenoceptor blocking properties of an array of 5-HT receptor ligands in the rat. - PubMed - NCBI
Agonism at 5-HT2B receptors is not a class effect of the ergolines. - PubMed - NCBI
Mechanism of decrease in heart rate by peripheral dopaminergic D2-receptors. - PubMed - NCBI
Studies on the inhibition of adrenaline-induced aggregation of blood platelets. - PubMed - NCBI
Fibrosis/Cancer
[Treatment of fibrocystic breast disease with lisuride]. - PubMed - NCBI
Metabolism/Diabetes/Cortisol/Insulin/Obesity
[The effect of noradrenaline and dopamine agonists and antagonists on oxygen consumption in some insect and crustacean species]. - PubMed - NCBI
[Medical treatment of Cushing syndrome]. - PubMed - NCBI
[Stimulatory effects of lisuride on local cerebral blood flow and local cerebral glucose utilization in rats]. - PubMed - NCBI
Dopaminergic modulation of aldosterone secretion in the normal menstrual cycle. - PubMed - NCBI
Dopamine control of aldosterone secretion in end-stage renal failure. - PubMed - NCBI
Alpha-1 adrenergic blockade: a possible mechanism of action of dopaminergic drugs on ACTH secretion. - PubMed - NCBI
Effect of short and long-term administration of lisuride in Cushing's disease. - PubMed - NCBI
An in vitro stimulatory effect of indoleamines on aldosterone biosynthesis in the rat. - PubMed - NCBI
Anorectic effect of lisuride and other ergot derivatives in the rat. - PubMed - NCBI
Hypothermic action of lisuride in rats and differences to bromocriptine in the antagonistic effect of neuroleptics. - PubMed - NCBI
Immune/Antirival/Antibacterial/Antiparasitic
[Is dopaminergic therapy immunologically rejuvinating? Increased interferon-gamma production with the dopaminergic agent lisuride]. - PubMed - NCBI
5-HT7 receptor - Wikipedia, the free encyclopedia
An inactivaing antagonist renders a receptor persistently unable to react to an agonist such as serotonin or a serotonergic drug. This is similar to the effects of "suicidal" aromatase inhibitors like exemestane, which permanently deactivate the aromatase enzyme. These properties of lisuride suggest that it can have a very long-term serotonin antagonistic effects on specific receptors, and even a single dose can manifest its effects long after the lisuride is excreted from the body. This makes lisuride able to achieve beneficial effects even with very infrequent dosing, which has been confirmed in clinical trials at least in regards to lowering prolactin.
Lisuride - Wikipedia, the free encyclopedia
"...Lisuride is used to lower prolactin and, in low doses, to prevent migraine attacks. The use of lisuride as initial anti-Parkinsonian treatment has been advocated, delaying the need for levodopa until lisuride becomes insufficient for controlling the parkinsonian disability. Preliminary trials suggest the dermal application of lisuride may be useful in the treatment of Parkinson's disease. As lisuride is very poorly absorbed when take orally and has a short half-life, continuous transdermal administration offers significant advantages and could make the compound a far more consistent therapeutic. Lisuride is not currently available in the US, as the drug was not a commercial success in comparison with other dopamine receptor agonist antiparkinsonian compounds."
The units listed on the label are just for measurement purposes. They do not indicate suggested or optimal dose. Please note that similar to the products sold by companies like BlueSky, this product if for lab/research use only. The product can be ordered from the link below:
IdeaLabs Online Store - Worldwide Ordering And Delivery - Laboratory Research Chemicals
*******************************************************************************
Lisuride is a chemical agent of the iso-ergoline class, related to the dopaminergic ergoline Parkinson's drugs. Lisuride is a full dopamine and a mixed agonist/antagonist for several serotonin receptors. It is an antagonist at the serotonin 5-HT2B receptor, which is its main mechanism of action in reversing fibrosis of various tissues and organs. It has a high affinity for the dopamine D2, D3 and D4 receptors, as well as D1, and D5. It is an agonist of 5-HT1Aand 5-HT2C receptors, and putative antagonist on 5-HT2A (which explains its antagonism to LSD hallucinogenic effects). Lisuride is also a putative histamine antagonist, on both H1 and H2 receptors.
Drops per container: about 240
Each drop contains the following ingredients:
Lisuride (maleate): 25 mcg
Other ingredients: add product to shopping cart to see info
Note: Liquid preparations of lisuride are best kept at a temp of 20 degrees C or lower to ensure shelf-life of at least 3 months.
*******************************************************************************
References:
Miscellaneous
Influence of lisuride, a dopaminergic agonist, on the sexual function of male patients with chronic renal failure. - PubMed - NCBI
"...The increase of plasma testosterone was greater in hyperprolactinemic patients (86% v 15% in normoprolactinemic) and was accompanied by a clear improvement in the studied parameters of sexual behaviors. The response of PRL to TRH was modified in hyperprolactinemic patients while that of LH and FSH to LH-RH was not modified, although Lisuride induced an increase of the basal value of LH (P less than 0.01) in the hyperprolactinemic group. The drug was fairly well tolerated, did not induce hypotension, and the overall incidence of side effects decreased along the study."
Pharmacological analysis of the novel, rapid, and potent inactivation of the human 5-Hydroxytryptamine7 receptor by risperidone, 9-OH-Risperidone, ... - PubMed - NCBI
Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. - PubMed - NCBI
Pharmacological properties of a wide array of ergolines at functional alpha(1)-adrenoceptor subtypes. - PubMed - NCBI
In vitro pharmacology of clinically used central nervous system-active drugs as inverse H(1) receptor agonists. - PubMed - NCBI
Glutamate-induced cell death and formation of radicals can be reduced by lisuride in mesencephalic primary cell culture. - PubMed - NCBI
Mastalgia: a review of management. - PubMed - NCBI
Involvement of 5-hydroxytryptamine (HT)7 receptors in the 5-HT excitatory effects on the rat urinary bladder. - PubMed - NCBI
No evidence of the usefulness of eye blinking as a marker for central dopaminergic activity. - PubMed - NCBI
Re-evaluation of lisuride pharmacology: 5-hydroxytryptamine1A receptor-mediated behavioral effects overlap its other properties in rats. - PubMed - NCBI
Lisuride acts at multiple sites to induce ocular hypotension and mydriasis. - PubMed - NCBI
Agonist activity of LSD and lisuride at cloned 5HT2A and 5HT2C receptors. - PubMed - NCBI
Specific involvement of central 5-HT1A receptors in the mediation of male rat ejaculatory behavior. - PubMed - NCBI
The effects of lisuride, terguride and bromocriptine on intraocular pressure (IOP). - PubMed - NCBI
Comparative effects of LSD and lisuride: clues to specific hallucinogenic drug actions. - PubMed - NCBI
Antagonism of L-5-hydroxytryptophan-induced head twitching in rats by lisuride: a mixed 5-hydroxytryptamine agonist-antagonist? - PubMed - NCBI
Influence of lisuride, a dopaminergic agonist, on the sexual function of male patients with chronic renal failure. - PubMed - NCBI
Serotonin involvement in lisuride-induced mounting and in sleep. - PubMed - NCBI
Stimulation of dopamine autoreceptors elicits "premature ejaculation" in rats. - PubMed - NCBI
Lisuride, LY-141865, and 8-OH-DPAT facilitate male rat sexual behavior via a non-dopaminergic mechanism. - PubMed - NCBI
Spinal control of sexual behavior: effects of intrathecal administration of lisuride. - PubMed - NCBI
Spinal control of sexual behavior: Effects of intrathecal administration of lisuride - ScienceDirect
Lysergic acid diethylamide (LSD) and lisuride: differentiation of their neuropharmacological actions. - PubMed - NCBI
Serotonergic and dopaminergic effects of yawning in the cat. - PubMed - NCBI
The interaction of lisuride, an ergot derivative, with serotonergic and dopaminergic receptors in rabbit brain. - PubMed - NCBI
Inhibition of neurotransmitter receptor binding by ergot derivatives. - PubMed - NCBI
Stimulating effects of lisuride on masculine sexual behavior of rats. - PubMed - NCBI
Induction of mounting behaviour in female and male rats by lisuride. - PubMed - NCBI
Neurological/Brain
Lisuride reduces involuntary periodic leg movements in spinocerebellar ataxia type 2 patients. - PubMed - NCBI
Drug Treatments for the Prevention of Migraine Headache - PubMed - NCBI
Anticonvulsive effects of the dopamine agonist lisuride maleate after experimental traumatic brain injury. - PubMed - NCBI
Lisuride treatment of restless legs syndrome: first studies with monotherapy in de novo patients and in combination with levodopa in advanced disease. - PubMed - NCBI
Transdermal lisuride: short-term efficacy and tolerability study in patients with severe restless legs syndrome. - PubMed - NCBI
Transdermal lisuride delivery in the treatment of Parkinson's disease. - PubMed - NCBI
The dopamine receptor agonist lisuride attenuates iron-mediated dopaminergic neurodegeneration. - PubMed - NCBI
Protection of dopaminergic neurons in primary culture by lisuride. - PubMed - NCBI
Lisuride treatment of Alzheimer's disease. A preliminary placebo-controlled clinical trial of safety and therapeutic efficacy. - PubMed - NCBI
Lisuride prevents learning and memory impairment and attenuates the increase in extracellular dopamine induced by transient global cerebral ischemi... - PubMed - NCBI
Neuroprotection by dopamine agonists. - PubMed - NCBI
[Effect of lisuride on experimental cerebral infarction in rats]. - PubMed - NCBI
Antagonist effect of terguride in Parkinson's disease. - PubMed - NCBI
Chronic lisuride hydrogen maleate administration enhances muscarinic receptor binding in senescent rat brain. - PubMed - NCBI
Intravenous administration of lisuride in the treatment of neuroleptic malignant syndrome. - PubMed - NCBI
[Lisuride for the prevention of migraine. Results of a multicenter study]. - PubMed - NCBI
Effects of lisuride hydrogen maleate on ischemia-induced depletion of brain acetylcholine levels in gerbils. - PubMed - NCBI
Low dose lisuride in advanced Parkinson disease. - PubMed - NCBI
[Analysis of the clinical experience with 136 patients with headache treated with lisuride]. - PubMed - NCBI
Acute treatment of Huntington's chorea with lisuride. - PubMed - NCBI
Beneficial effects of lisuride in Meige disease. - PubMed - NCBI
The use of lisuride, a potent dopamine and serotonin agonist, in the treatment of progressive supranuclear palsy. - PubMed - NCBI
Dopamine agonists and cobalt-induced epilepsy in the rat. - PubMed - NCBI
Lisuride, a potent drug in the treatment of muscular rigisity in rats. - PubMed - NCBI
Mood/Cognition/Behavior/Addiction
[Treatment of premenstrual tension syndrome (PMS) with lisuride maleate]. - PubMed - NCBI
The dopamine agonists lisuride and piribedil protect against behavioural and histological changes following 4-vessel occlusion in the rat. - PubMed - NCBI
Striatal dopamine receptors and adenylyl cyclase activity in a rat model of alcohol addiction: effects of ethanol and lisuride treatment. - PubMed - NCBI
The effects of lisuride on mood and sleep during acute withdrawal in stimulant abusers: a preliminary report. - PubMed - NCBI
Lisuride reduces intravenous cocaine self-administration in rats. - PubMed - NCBI
[Therapeutic effect of lisuride maleate on post-stroke depression]. - PubMed - NCBI
Lisuride reduces psychomotor retardation during withdrawal from chronic intravenous amphetamine self-administration in rats. - PubMed - NCBI
Treating organic abulia with bromocriptine and lisuride: four case studies. - PubMed - NCBI
[Anxiolytic effects of lisuride and its agonistic action to central 5-HT1A receptors]. - PubMed - NCBI
[Effects in animal models of depression of lisuride alone and upon coadministration with antidepressants]. - PubMed - NCBI
Recovery of memory following forgetting induced by depletion of biogenic amines. - PubMed - NCBI
CVD/Circulation
Fibrotic heart-valve reactions to dopamine-agonist treatment in Parkinson's disease. - PubMed - NCBI
Lisuride, a dopamine receptor agonist with 5-HT2B receptor antagonist properties: absence of cardiac valvulopathy adverse drug reaction reports sup... - PubMed - NCBI
Potential vascular alpha1-adrenoceptor blocking properties of an array of 5-HT receptor ligands in the rat. - PubMed - NCBI
Agonism at 5-HT2B receptors is not a class effect of the ergolines. - PubMed - NCBI
Mechanism of decrease in heart rate by peripheral dopaminergic D2-receptors. - PubMed - NCBI
Studies on the inhibition of adrenaline-induced aggregation of blood platelets. - PubMed - NCBI
Fibrosis/Cancer
[Treatment of fibrocystic breast disease with lisuride]. - PubMed - NCBI
Metabolism/Diabetes/Cortisol/Insulin/Obesity
[The effect of noradrenaline and dopamine agonists and antagonists on oxygen consumption in some insect and crustacean species]. - PubMed - NCBI
[Medical treatment of Cushing syndrome]. - PubMed - NCBI
[Stimulatory effects of lisuride on local cerebral blood flow and local cerebral glucose utilization in rats]. - PubMed - NCBI
Dopaminergic modulation of aldosterone secretion in the normal menstrual cycle. - PubMed - NCBI
Dopamine control of aldosterone secretion in end-stage renal failure. - PubMed - NCBI
Alpha-1 adrenergic blockade: a possible mechanism of action of dopaminergic drugs on ACTH secretion. - PubMed - NCBI
Effect of short and long-term administration of lisuride in Cushing's disease. - PubMed - NCBI
An in vitro stimulatory effect of indoleamines on aldosterone biosynthesis in the rat. - PubMed - NCBI
Anorectic effect of lisuride and other ergot derivatives in the rat. - PubMed - NCBI
Hypothermic action of lisuride in rats and differences to bromocriptine in the antagonistic effect of neuroleptics. - PubMed - NCBI
Immune/Antirival/Antibacterial/Antiparasitic
[Is dopaminergic therapy immunologically rejuvinating? Increased interferon-gamma production with the dopaminergic agent lisuride]. - PubMed - NCBI
Last edited: