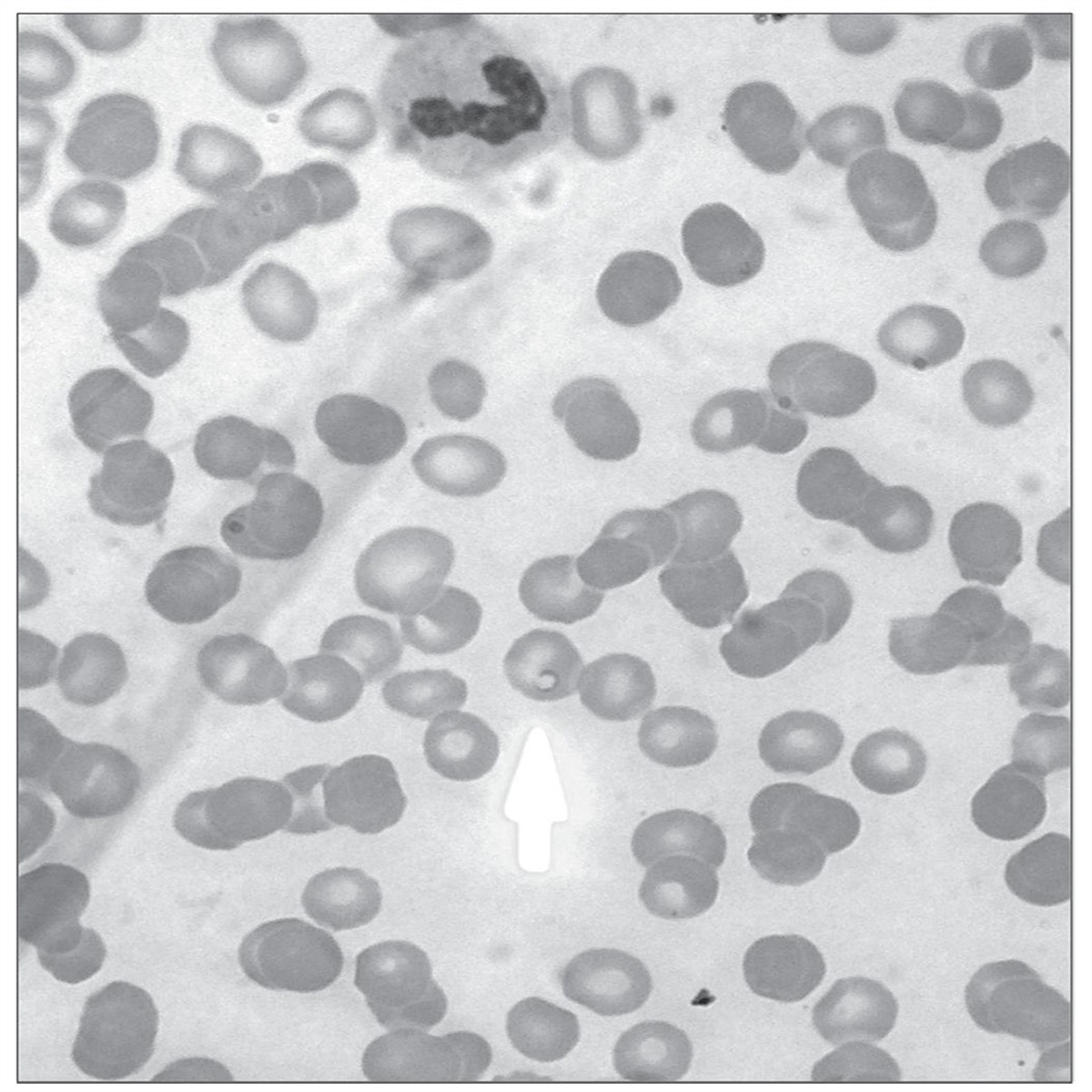
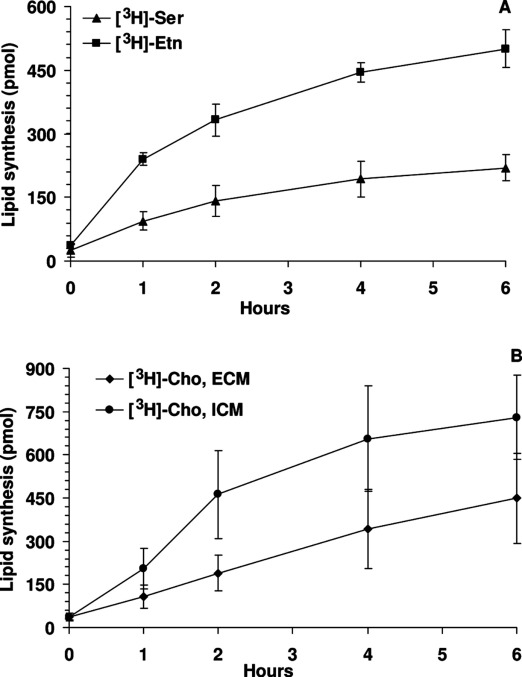
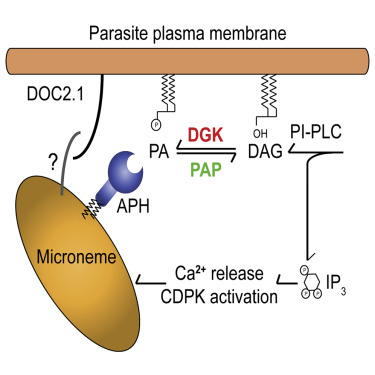
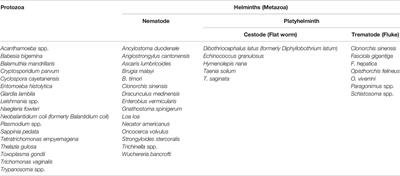
I would not wonder if an underlying weak spot of them or the way they hijack tissue would create a nice anti apicomplexa framework of substances, and or treatments (methylene blue + red light)....Apparently t gondii is a part of a diverse group of parasites called apicomplexans. Here is their theorized evolutionary tree: Apicomplexa - Wikipedia
Diseases caused by Apicomplexa include: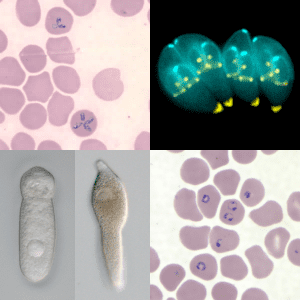
Apicomplexa - Wikipedia
en.wikipedia.org
- Babesiosis (Babesia)
- Malaria (Plasmodium)
- Cryptosporidiosis (Cryptosporidium parvum)
- Cyclosporiasis (Cyclospora cayetanensis)
- Cystoisosporiasis (Cystoisospora belli (formerly known as "Isospora Belli"))
- Toxoplasmosis (Toxoplasma gondii)
Maybe they also share a common path for their abilities in immune suppression which could be reversed and so any problems with 'em fixed.