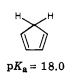Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
Correct, I should have said that but I meant it as all one step, where the radical absconds the hydrogen with an electron and replaces it with an O2. Thanks for the info, it is hard to find these basic measurements that should be available sometimes, isn't it?
I found this, which already has units of energy.
I suppose you can work backwards to see what pKa this gives, to see of the 9⋅kcal/mol simply represents the hydrogen abstraction. I would expect a value of between 15 and 25 since the pKa of 1,3‐cyclopentadiene is often heuristically stated as 'the most acidic diene' and has a value of ~18.⁽¹⁾ This radical is stabilized by resonance, making it more acid than most diene carbon acids. An article on olefins gives a range of 10–17,⁽²⁾ so the pKa should be something like that.. . .
But it is only at 7.20? Better check his source [10].⁽³⁾
'Hydrogen abstraction from a bis-allylic methylene group by alkoxyl and alkylperoxyl radicals is favourable with Gibbs energies of -23 and -9 kcal/mol, respectively.' ―Koppenol⁽³⁾
The other value yields a pKa of 18.40,* which is in the range of olefins⁽²⁾ and close to that of 1,3 cyclopentadiene.⁽¹⁾ The energy of the alkoxyl abstraction (Lipid₁–O· + H–Lipid₂) of −23⋅kcal/mol seems close to the spontaneous loss of the initial central hydrogen (pKa) of dienes in water.
'Thermodynamically, the hydrodioxyl radical should also be able to oxidize the singly allylic hydrogen in oleic acid, but no evidence for this reaction has been found [15].' ―Koppenol⁽³⁾
So the energies involved in the chain reaction seem slightly different than the initial loss of the first hydrogen to water, unless this event happens to be characterized by abstraction with a rogue hydroxyl radical (H–O·). I would think this would be similar in energy to that of the alkoxy radical (Lipid₁–O·) abstraction of −23⋅kcal/mol since they are both single oxygen radicals. Of course, hydroxyl radicals can be formed through Fenton reactions and are thought to be common molecules within the cell (especially in those supplementing with iron, eating refined wheat flour, or low in antioxidants.)
'The data in Tables I and II indicate that an alkylperoxyl radical cannot abstract a non-allylic hydrogen from a hydrocarbon, ΔG = + 12 kcal.' ―Koppenol⁽³⁾
The oxidation of saturated fatty acids is thermodynamically unfavorable, making coconuts a great choice.
[2] Hegedus, Louis S. "Palladium-assisted alkylation of olefins." Journal of the American Chemical Society (1980)
[3] Koppenol, W. H. "Oxyradical reactions: from bond‐dissociation energies to reduction potentials." FEBS letters (1990)
[†] Min, B. "Mechanism of lipid peroxidation in meat and meat products-A review." Food Science and Biotechnology (2005)
[*] First, to go from calories to joules:
[3] Koppenol, W. H. "Oxyradical reactions: from bond‐dissociation energies to reduction potentials." FEBS letters (1990)
[†] Min, B. "Mechanism of lipid peroxidation in meat and meat products-A review." Food Science and Biotechnology (2005)
[*] First, to go from calories to joules:
(−9⋅kcal/mol)×(4.184⋅J/cal) = −37.656⋅kJ/mol
Then the Gibbs Free Energy equation solving for Ka:ΔG = −R⋅T⋅lnKa
(−ΔG/R⋅T) = lnKa
(−37.656·kJ/mol)/(8.3144598⋅J⋅mol⁻¹⋅K⁻¹)⋅(273.15) = lnKa
The standard temperature in Kelvin is 273.15 degrees, and 'R' is Boltzmann's constant. (−ΔG/R⋅T) = lnKa
(−37.656·kJ/mol)/(8.3144598⋅J⋅mol⁻¹⋅K⁻¹)⋅(273.15) = lnKa
(−37.656·kJ/mol)/(8.3144598⋅J⋅mol⁻¹⋅K⁻¹)⋅(273.15°K) = lnKa
(−37.656·kJ)/(8.3144598⋅J)⋅(273.15) = lnKa
(−37.656·kJ)/(8.3144598⋅J)⋅(273.15) = lnKa
(16.581) = lnKa
ℯ¹⁶⋅⁵⁸¹ = Ka
15886813 = Ka
log(15886813) = pKa
7.20 = pKa = when starting with the value of −9⋅kcal/mol
18.40 = pKa = when starting with the value of −23⋅kcal/mol
(−37.656·kJ)/(8.3144598⋅J)⋅(273.15) = lnKa
(−37.656·kJ)/(8.3144598⋅J)⋅(273.15) = lnKa
(16.581) = lnKa
ℯ¹⁶⋅⁵⁸¹ = Ka
15886813 = Ka
log(15886813) = pKa
7.20 = pKa = when starting with the value of −9⋅kcal/mol
18.40 = pKa = when starting with the value of −23⋅kcal/mol
Last edited: