Obi-wan
Member
- Joined
- Mar 16, 2017
- Messages
- 1,120
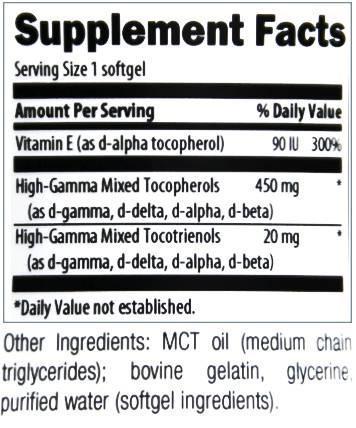
Picture would not display . Has High Gamma Tocotrienols 20mgs
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.

Gamma and delta tocopherol are toxic in ways alpha tocopherol isn't, according to this paper.
The cytotoxicity of vitamin E is both vitamer- and cell-specific and involves a selectable trait.
During a study of the effect of vitamin E in activated mouse macrophages, we observed a reduction in the viability of cells treated with various forms of vitamin E. We show in this report that some tocopherols (both gamma- and delta-tocopherol) are cytotoxic to some but not all cell types. Mouse macrophages were especially sensitive (40 micromol/L), whereas human hepatocytes and bovine endothelial cells were almost completely refractory (90 micromol/L). The fully methylated tocopherol, alpha-tocopherol (alpha-Toc), was not cytotoxic in any cell type tested. The cytotoxicity observed with delta-tocopherol (delta-Toc) was associated with 2 markers of apoptosis. Vitamer-specific cytotoxicity was not due to differences in cellular uptake/accumulation because both alpha-Toc and delta-Toc accumulated equally in any cell type tested. In contrast, the cell-specific cytotoxicity was related in part to uptake/accumulation of the tocopherols. Macrophages accumulated nearly 5 times more tocopherol compared with hepatocytes cultured under similar conditions. To address the hypothesis that uptake accounted for the cell-specific sensitivity, we developed a macrophage "subtype" that was markedly resistant (>150 micromol/L) to delta-Toc. Under many different cell culture conditions (including human serum) uptake/accumulation of tocopherols was reduced in this subtype by approximately 50%. Further selection and evaluation of this phenotype, however, demonstrated no cytotoxicity even when cellular levels were elevated. Our results show that undermethylated tocopherols are cytotoxic to macrophages and that there are independent and selectable processes that determine cellular tocopherol uptake/accumulation and delta-Toc cytotoxicity.
Oh well, maybe there is a point where keeping taking mixed tocopherols is bad, and we don't know what that is.
Perhaps this is relevant to the discussion:
"We show in this report that some tocopherols (both gamma- and delta-tocopherol) are cytotoxic to some but not all cell types." - if it is cancer cells that is great...
It has been shown that circulating oleic acid will enter the brain, and I think oleamide could partialy explain 'Italian psychology:' dramatic behavior, over-emotionality, impulsiveness, rudeness, and absurd language are all Italian archetypes (see Goodfellas);⁽¹⁾⁽²⁾⁽³⁾ the list of famous scientists who claim Italy as a homeland is disproportionately small. But what the high-sertotonin brain lacks in logic it can make-up for in poetry, music, and art. (I'm a German descendant myself, and like the Peter Duesberg's personality much more than his unscrupulous Italian rival: Robert Gallo.)
Fun post - and of course Duesberg > Gallo, since Duesberg has contributed, and Gallo is a fraud.
I find the reductionism of oleamide causing 'Italian behavior' to be significantly more extreme than many of the central dogma of biology.
Neither Travolta nor Olivia Newton John claim to have Italian ancestry...How else can one even begin to explain these people?
Doesn't his name, face, and manner of speech obviously give that away?Neither Travolta nor Olivia Newton John claim to have Italian ancestry...
It's founded on freely available and easy-to-find published lists of scientists, as well as memory, yet that remark had obviously been somewhat facetious.Was this remark founded in data or merely instinctual?
The point is that oleamide has been shown to potentiate three distinct serotonin receptor subtypes at nanomolar concentrations, and with great effect, making this molecule arguably more serotonergic than serotonin itself. It does not antagonize indole ligands yet binds allosterically to an adjacent site. While true that it's powerful sleep promoting effects in rats and cats may not be shared with humans, there is also a good deal of interspecies variation in the distribution of: melatonin receptors, serotonin receptors, and endocannibinoid receptors as well as the respective G proteins they're coupled to. Nonetheless: oleamide can still be expected to potentiate three separate human 5-hydroxytryptophan receptors in nanomolar concentrations, regardless of whether or not they're associated with sleep promoting brain regions.
I had posted this somewhere else.How else can one even begin to explain these people?
Wouldn't that explain sleep deprivation's effect on depression though?
I'm not sure I would associate serotonin with joy, libido and dancing, but I don't know much.
I had posted this somewhere else.

Okay: Perhaps John Travolta could have been 'just acting,' but is there any other way to explain Frankie Vallie?

Thanks for the responseIMO high gamma Vit. E mixed Tocopherols in MCT oil and dry e succinate
But this kills all cells, right? My justification for taking γ-tocopherol is that it adducts with peroxynitrite and nitrogen dioxide, which are powerful mutagens shown to initiate cancer. There's a considerable amount of evidence for γ-tocopherol preventing cancer, yet it may not be the most effective thing for reversing it. I had just read a considerable amount about sodium, potassium, cell membrane potential, and mitosis. Sodium is actually considered a mitotic growth factor by Clarence Cone—head of the Biochemistry & Biophysics Department at NASA's Langley Research Center—and many others.@Travis can ROS and RNS be a good thing for cancer in killing it? See https://cancerandmetabolism.biomedcentral.com/articles/10.1186/2049-3002-2-17. Maybe I should not be doing any Vit. E...
