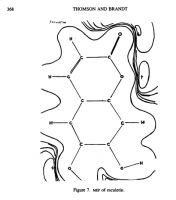
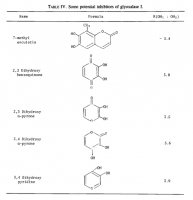
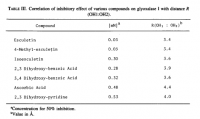
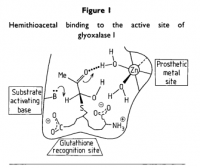
Amazoniac
Member
I was reading something about it and things didn't seem to connect. Wikipedia claims Tabebuia avellanedae and impetiginosa are the same thing for example, but apparently they're not. I have found studies comparing the development of a seed from one plant and the other, so of course they can't be the same.
And then I came across this.
There are visible variances in some of these plants, so perhaps the bark varies as well. It's difficult after viewing such list to not question what makes impetiginosa special to be considered the best option out of those.
@healthnatura @LifeGivingStore
And then I came across this.
Tabebuia acrophylla
Tabebuia actinophylla
Tabebuia acunana
Tabebuia aesculifolia
Tabebuia affinis
Tabebuia alba
Tabebuia anafensis
Tabebuia angustata
Tabebuia anisophylla
Tabebuia apiculata
Tabebuia aquatilis
Tabebuia araliacea
Tabebuia arenicola
Tabebuia argentea
Tabebuia arianeae
Tabebuia arimaoensis
Tabebuia atrovirens
Tabebuia aurea
Tabebuia avellanedae
Tabebuia bahamensis
Tabebuia barbata
Tabebuia berterii
Tabebuia berteroi
Tabebuia beyeri
Tabebuia bibracteolata
Tabebuia billbergii
Tabebuia botelhensis
Tabebuia brevipes
Tabebuia brigandina
Tabebuia brooksiana
Tabebuia buchii
Tabebuia bullata
Tabebuia bureauvii
Tabebuia bureavii
Tabebuia calcicola
Tabebuia calderoni
Tabebuia calderonii
Tabebuia caleticana
Tabebuia camagueyensis
Tabebuia candicans
Tabebuia capitata
Tabebuia capotei
Tabebuia caraiba
Tabebuia cassinoides
Tabebuia catarinensis
Tabebuia chapadensis
Tabebuia chrysantha
Tabebuia chrysea
Tabebuia chrysotricha
Tabebuia citrifolia
Tabebuia clementis
Tabebuia coartata
Tabebuia conferta
Tabebuia coralibe
Tabebuia cordata
Tabebuia cowellii
Tabebuia crassifolia
Tabebuia crispiflora
Tabebuia cristata
Tabebuia cuneifolia
Tabebuia curtissii
Tabebuia del [what?]
Tabebuia densifolia
Tabebuia dentata
Tabebuia dictyophylla
Tabebuia diluvialis
Tabebuia dolichopoda
Tabebuia domingensis
Tabebuia dominicensis
Tabebuia donell-smithii
Tabebuia donnell
Tabebuia dracocephaloides
Tabebuia dubia
Tabebuia dugandii
Tabebuia dura
Tabebuia ecuadorensis
Tabebuia ekmanii
Tabebuia elegans
Tabebuia elliptica
Tabebuia elongata
Tabebuia erosa
Tabebuia excisa
Tabebuia eximia
Tabebuia fallax
Tabebuia flavescens
Tabebuia floccosa
Tabebuia fluviatilis
Tabebuia furfuracea
Tabebuia fuscata
Tabebuia gemmiflora
Tabebuia geronensis
Tabebuia glaucescens
Tabebuia globiflora
Tabebuia glomerata
Tabebuia gonavensis
Tabebuia gracilipes
Tabebuia grisebachii
Tabebuia guayacan
Tabebuia haemantha
Tabebuia heptaphylla
Tabebuia heterophylla
Tabebuia heteropoda
Tabebuia heterotricha
Tabebuia hotteana
Tabebuia hypodictyon
Tabebuia hypolepra
Tabebuia hypoleuca
Tabebuia ilicifolia
Tabebuia imisspboy
Tabebuia impetiginosa
Tabebuia inaequipes
Tabebuia incana
Tabebuia insignis
Tabebuia insignis
Tabebuia ipe
Tabebuia jackiana
Tabebuia jamaicensis
Tabebuia japurensis
Tabebuia jaucoensis
Tabebuia jojoana
Tabebuia lanceolata
Tabebuia lapacho
Tabebuia latifolia
Tabebuia leonis
Tabebuia lepidophylla
Tabebuia lepidota
Tabebuia lepidota
Tabebuia leptoneura
Tabebuia leptopoda
Tabebuia leucoxyla
Tabebuia libanensis
Tabebuia lindahlii
Tabebuia linearis
Tabebuia litoralis
Tabebuia longiflora
Tabebuia longipes
Tabebuia lopezii
Tabebuia lucida
Tabebuia maestrensis
Tabebuia magnolioides
Tabebuia mansoana
Tabebuia maxonii
Tabebuia mexicana
Tabebuia micrantha
Tabebuia microphylla
Tabebuia millsii
Tabebuia moaensis
Tabebuia mogotensis
Tabebuia multinervis
Tabebuia myrtifolia
Tabebuia neochrysantha
Tabebuia nervosa
Tabebuia neurophylla
Tabebuia nicaraguensis
Tabebuia nigripes
Tabebuia nipensis
Tabebuia nivea
Tabebuia nodosa
Tabebuia nodosa
Tabebuia obovata
Tabebuia obscura
Tabebuia obtusifolia
Tabebuia ochracea
Tabebuia odontodiscus
Tabebuia oligolepis
Tabebuia ophiolithica
Tabebuia ophiticola
Tabebuia orinocensis
Tabebuia ostenfeldii
Tabebuia ovatifolia
Tabebuia pachyphylla
Tabebuia pallida
Tabebuia palmeri
Tabebuia palustris
Tabebuia paniculata
Tabebuia papyrophloios
Tabebuia pedicellata
Tabebuia pentaphylla
Tabebuia perelegans
Tabebuia perfae
Tabebuia pergracilis
Tabebuia petrophila
Tabebuia picotensis
Tabebuia pilosa
Tabebuia pinetorum
Tabebuia pisoniana
Tabebuia piutinga
Tabebuia platyantha
Tabebuia polyantha
Tabebuia polymorpha
Tabebuia potamophila
Tabebuia pulcherrima
Tabebuia pulverulenta
Tabebuia pumila
Tabebuia punctatissima
Tabebuia pyramidata
Tabebuia reticulata
Tabebuia revoluta
Tabebuia ricardii
Tabebuia richardiana
Tabebuia rigida
Tabebuia riodocensis
Tabebuia riparia
Tabebuia roraimae
Tabebuia rosea
Tabebuia roseo
Tabebuia roseo-alba
Tabebuia rubriflora
Tabebuia rufescens
Tabebuia sauvallei
Tabebuia schumanniana
Tabebuia serratifolia
Tabebuia striata
Tabebuia umbellata
Tabebuia vellosoi
Tabebuia actinophylla
Tabebuia acunana
Tabebuia aesculifolia
Tabebuia affinis
Tabebuia alba
Tabebuia anafensis
Tabebuia angustata
Tabebuia anisophylla
Tabebuia apiculata
Tabebuia aquatilis
Tabebuia araliacea
Tabebuia arenicola
Tabebuia argentea
Tabebuia arianeae
Tabebuia arimaoensis
Tabebuia atrovirens
Tabebuia aurea
Tabebuia avellanedae
Tabebuia bahamensis
Tabebuia barbata
Tabebuia berterii
Tabebuia berteroi
Tabebuia beyeri
Tabebuia bibracteolata
Tabebuia billbergii
Tabebuia botelhensis
Tabebuia brevipes
Tabebuia brigandina
Tabebuia brooksiana
Tabebuia buchii
Tabebuia bullata
Tabebuia bureauvii
Tabebuia bureavii
Tabebuia calcicola
Tabebuia calderoni
Tabebuia calderonii
Tabebuia caleticana
Tabebuia camagueyensis
Tabebuia candicans
Tabebuia capitata
Tabebuia capotei
Tabebuia caraiba
Tabebuia cassinoides
Tabebuia catarinensis
Tabebuia chapadensis
Tabebuia chrysantha
Tabebuia chrysea
Tabebuia chrysotricha
Tabebuia citrifolia
Tabebuia clementis
Tabebuia coartata
Tabebuia conferta
Tabebuia coralibe
Tabebuia cordata
Tabebuia cowellii
Tabebuia crassifolia
Tabebuia crispiflora
Tabebuia cristata
Tabebuia cuneifolia
Tabebuia curtissii
Tabebuia del [what?]
Tabebuia densifolia
Tabebuia dentata
Tabebuia dictyophylla
Tabebuia diluvialis
Tabebuia dolichopoda
Tabebuia domingensis
Tabebuia dominicensis
Tabebuia donell-smithii
Tabebuia donnell
Tabebuia dracocephaloides
Tabebuia dubia
Tabebuia dugandii
Tabebuia dura
Tabebuia ecuadorensis
Tabebuia ekmanii
Tabebuia elegans
Tabebuia elliptica
Tabebuia elongata
Tabebuia erosa
Tabebuia excisa
Tabebuia eximia
Tabebuia fallax
Tabebuia flavescens
Tabebuia floccosa
Tabebuia fluviatilis
Tabebuia furfuracea
Tabebuia fuscata
Tabebuia gemmiflora
Tabebuia geronensis
Tabebuia glaucescens
Tabebuia globiflora
Tabebuia glomerata
Tabebuia gonavensis
Tabebuia gracilipes
Tabebuia grisebachii
Tabebuia guayacan
Tabebuia haemantha
Tabebuia heptaphylla
Tabebuia heterophylla
Tabebuia heteropoda
Tabebuia heterotricha
Tabebuia hotteana
Tabebuia hypodictyon
Tabebuia hypolepra
Tabebuia hypoleuca
Tabebuia ilicifolia
Tabebuia imisspboy
Tabebuia impetiginosa
Tabebuia inaequipes
Tabebuia incana
Tabebuia insignis
Tabebuia insignis
Tabebuia ipe
Tabebuia jackiana
Tabebuia jamaicensis
Tabebuia japurensis
Tabebuia jaucoensis
Tabebuia jojoana
Tabebuia lanceolata
Tabebuia lapacho
Tabebuia latifolia
Tabebuia leonis
Tabebuia lepidophylla
Tabebuia lepidota
Tabebuia lepidota
Tabebuia leptoneura
Tabebuia leptopoda
Tabebuia leucoxyla
Tabebuia libanensis
Tabebuia lindahlii
Tabebuia linearis
Tabebuia litoralis
Tabebuia longiflora
Tabebuia longipes
Tabebuia lopezii
Tabebuia lucida
Tabebuia maestrensis
Tabebuia magnolioides
Tabebuia mansoana
Tabebuia maxonii
Tabebuia mexicana
Tabebuia micrantha
Tabebuia microphylla
Tabebuia millsii
Tabebuia moaensis
Tabebuia mogotensis
Tabebuia multinervis
Tabebuia myrtifolia
Tabebuia neochrysantha
Tabebuia nervosa
Tabebuia neurophylla
Tabebuia nicaraguensis
Tabebuia nigripes
Tabebuia nipensis
Tabebuia nivea
Tabebuia nodosa
Tabebuia nodosa
Tabebuia obovata
Tabebuia obscura
Tabebuia obtusifolia
Tabebuia ochracea
Tabebuia odontodiscus
Tabebuia oligolepis
Tabebuia ophiolithica
Tabebuia ophiticola
Tabebuia orinocensis
Tabebuia ostenfeldii
Tabebuia ovatifolia
Tabebuia pachyphylla
Tabebuia pallida
Tabebuia palmeri
Tabebuia palustris
Tabebuia paniculata
Tabebuia papyrophloios
Tabebuia pedicellata
Tabebuia pentaphylla
Tabebuia perelegans
Tabebuia perfae
Tabebuia pergracilis
Tabebuia petrophila
Tabebuia picotensis
Tabebuia pilosa
Tabebuia pinetorum
Tabebuia pisoniana
Tabebuia piutinga
Tabebuia platyantha
Tabebuia polyantha
Tabebuia polymorpha
Tabebuia potamophila
Tabebuia pulcherrima
Tabebuia pulverulenta
Tabebuia pumila
Tabebuia punctatissima
Tabebuia pyramidata
Tabebuia reticulata
Tabebuia revoluta
Tabebuia ricardii
Tabebuia richardiana
Tabebuia rigida
Tabebuia riodocensis
Tabebuia riparia
Tabebuia roraimae
Tabebuia rosea
Tabebuia roseo
Tabebuia roseo-alba
Tabebuia rubriflora
Tabebuia rufescens
Tabebuia sauvallei
Tabebuia schumanniana
Tabebuia serratifolia
Tabebuia striata
Tabebuia umbellata
Tabebuia vellosoi
There are visible variances in some of these plants, so perhaps the bark varies as well. It's difficult after viewing such list to not question what makes impetiginosa special to be considered the best option out of those.
@healthnatura @LifeGivingStore
Last edited: