
Malonate Promotes Adult Cardiomyocyte Proliferation and Heart Regeneration - PubMed
Inhibition of SDH by malonate promotes adult cardiomyocyte proliferation, revascularization, and heart regeneration via metabolic reprogramming. These findings support a potentially important new therapeutic approach for human heart failure.
Abstract
Background: Neonatal mouse cardiomyocytes undergo a metabolic switch from glycolysis to oxidative phosphorylation, which results in a significant increase in reactive oxygen species production that induces DNA damage. These cellular changes contribute to cardiomyocyte cell cycle exit and loss of the capacity for cardiac regeneration. The mechanisms that regulate this metabolic switch and the increase in reactive oxygen species production have been relatively unexplored. Current evidence suggests that elevated reactive oxygen species production in ischemic tissues occurs as a result of accumulation of the mitochondrial metabolite succinate during ischemia via succinate dehydrogenase (SDH), and this succinate is rapidly oxidized at reperfusion. Mutations in SDH in familial cancer syndromes have been demonstrated to promote a metabolic shift into glycolytic metabolism, suggesting a potential role for SDH in regulating cellular metabolism. Whether succinate and SDH regulate cardiomyocyte cell cycle activity and the cardiac metabolic state remains unclear.Methods: Here, we investigated the role of succinate and SDH inhibition in regulation of postnatal cardiomyocyte cell cycle activity and heart regeneration.
Results: Our results demonstrate that injection of succinate into neonatal mice results in inhibition of cardiomyocyte proliferation and regeneration. Our evidence also shows that inhibition of SDH by malonate treatment after birth extends the window of cardiomyocyte proliferation and regeneration in juvenile mice. Remarkably, extending malonate treatment to the adult mouse heart after myocardial infarction injury results in a robust regenerative response within 4 weeks after injury via promoting adult cardiomyocyte proliferation and revascularization. Our metabolite analysis after SDH inhibition by malonate induces dynamic changes in adult cardiac metabolism.
Conclusions: Inhibition of SDH by malonate promotes adult cardiomyocyte proliferation, revascularization, and heart regeneration via metabolic reprogramming. These findings support a potentially important new therapeutic approach for human heart failure.
Introduction
Cardiovascular disease remains the leading cause of death in the world.1 Both vascular damage and myocardial damage arise from acute cardiovascular events such as myocardial infarction (MI). The limited capacity of the adult heart to repair itself represents a major barrier in cardiovascular medicine and often leads to heart failure. In contrast, the neonatal mouse heart has the ability to regenerate after MI, with the newly formed cardiac tissue being derived primarily from the proliferation of the preexisting cardiomyocytes.2–4 During postnatal development, exposure to high levels of atmospheric oxygen after birth results in a metabolic switch in energy utilization from glycolysis to oxidative phosphorylation.5 This metabolic switch results in increased mitochondrial reactive oxygen species (ROS) production, causing cardiomyocyte DNA damage and contributing to the postnatal cardiomyocyte cell cycle arrest in mice.6 Thus, understanding the metabolic state of the mammalian heart after birth can lead to important insights into restoring adult cardiomyocyte cell cycle activity and subsequent regenerative abilities after injury.Recent studies have demonstrated that the metabolite succinate accumulates during ischemia, which is a conserved phenomenon across vertebrates.7–9 Different models suggest that succinate accumulation occurs either through reverse activity of the enzyme complex succinate dehydrogenase (SDH; also known as complex II) or via canonical tricarboxylic acid cycle.7,9 SDH activity plays a central role in succinate accumulation in the proposed models owing to its involvement in both the tricarboxylic acid cycle and the electron transport chain.10 Subsequently, on reperfusion, the high levels of accumulated succinate are rapidly metabolized into fumarate, which results in a burst of ROS production via reverse activity of mitochondrial complex I.7,11 More important, administration of the SDH competitive inhibitor malonate prevents the accumulation of succinate and the subsequent metabolization that increases ROS levels during ischemia/reperfusion injury, emphasizing the link between SDH and ROS production.7,11,12
SDH plays an important role in metabolism and cell cycle activity in that it is the first mitochondrial protein to be identified as a tumor suppressor.13 Mutations in SDH in familial cancer syndromes promote a metabolic shift into glycolysis that drives cell division.13–15 Metabolic reprogramming to glycolysis is essential during zebrafish heart regeneration, which is concomitant with a significant reduction in SDH activity as well.16 However, whether succinate and SDH activity directly contributes to the limited regenerative capability of the heart after injury is unknown. In this study, we aim to determine the role of succinate and SDH in regulation of postnatal cardiomyocyte cell cycle activity and heart regeneration.
[...]
Discussion
Systolic heart failure often occurs as a consequence of the inability of the adult mammalian heart to regenerate after injury such as MI. Models of mammalian endogenous heart regeneration provide an opportunity to identify new approaches to restore adult human heart regeneration.30 Lineage tracing studies demonstrated that proliferation of the preexisting cardiomyocytes is the main source of the newly formed functional myocardium during endogenous regeneration. Thus, stimulating adult cardiomyocyte proliferation represents an important target in regenerating the adult human heart after injury.The metabolic switch in energy utilization of the postnatal heart and the subsequent increase in ROS production has emerged as an important factor in loss of this regenerative response.6 The mechanisms that regulate this metabolic switch remain unclear. In this study, our results demonstrate a powerful link between succinate metabolism and SDH activity and the regenerative response of the mammalian heart. We demonstrate that high levels of succinate can induce cardiomyocyte DNA damage and inhibit cardiomyocyte proliferation and regeneration. More important, we demonstrate that inhibition of SDH activity by malonate can also restore a cardiac regenerative response in the adult heart by stimulating adult cardiomyocyte cell cycle reentry and revascularization, important hallmarks of endogenous heart regeneration. This regenerative effect is caused largely by SDH inhibition because Atpenin A5, a potent inhibitor of SDH, can recapitulate the regenerative effect of malonate. Furthermore, our analysis demonstrates that SDH inhibition induces metabolite level changes that are consistent with a metabolic shift from oxidative phosphorylation to glycolysis in the adult heart. Although we did not detect an increase in lactate levels, serial metabolite analysis at multiple time points can reveal the extent of the dynamic cardiac metabolic shift after SDH inhibition. The metabolic switch from aerobic respiration to glycolysis is implicated by previous studies defining SDH as a tumor suppressor in which SDH mutations result in a metabolic reprogramming to glycolysis, which promotes cancer growth.13–15
Malonate has been demonstrated to play a cardioprotective role in reperfusion injury by inhibiting reverse activity of SDH, which prevents succinate accumulation and the subsequent redox insult and cardiac damage.7 SDH inhibition by malonate for 2 weeks increased succinate levels, a consequence of the inhibition of oxidative phosphorylation.23–25 In contrast to reperfusion injury, our results demonstrate that malonate does not exhibit a cardioprotective role after MI. The progression in restoration of cardiac structure and function over time strongly suggests a stimulation of a regenerative response by malonate after infarction, rather than protection. Furthermore, we demonstrate that malonate treatment starting 1 week after MI promotes myocardial regeneration and functional improvement over time. Collectively, these results reveal a novel role for SDH in its ability to metabolically reprogram the adult heart to a regenerative state. This underscores the translational potential of SDH inhibition as a powerful metabolic target for promoting adult heart regeneration.
There is an emerging appreciation for the role of metabolism in controlling cell state. Adult neural stem cell activity changes from a quiescent to a proliferative state via a metabolic shift by a single metabolite.31 Similarly, metabolic reprogramming regulates macrophage function in response to different stimuli.32,33 A recent study demonstrated that metabolic reprogramming is required for cardiomyocyte proliferation during zebrafish heart regeneration.16 The potential metabolic targeting of multiple cell types by systemic administration of malonate explains the striking regenerative effect after adult MI. In this study, we demonstrate that SDH inhibition promotes adult cardiomyocyte proliferation and revascularization after injury. The overall impact of malonate on other cell types needs to be investigated further. For example, SDH inhibition by malonate has been shown to promote an anti-inflammatory state of macrophages after lipopolysaccharide stimulation.34 Thus, whether SDH inhibition regulates the inflammatory response after infarction remains unclear. In addition, mutations in SDH have been shown to promote DNA methylation, demonstrating an interplay between epigenetics and metabolism.14,23,35 Whether SDH inhibition by malonate can modulate the epigenetic landscape and the transcriptional activity of multiple cardiac cell types needs to be determined.
Understanding the effect of malonate on the heart and other tissues will be an essential step before our findings can be translated to the clinic for the treatment of heart failure. Targeted delivery to the heart would avoid any off-target effects from systemic SDH inhibition. Furthermore, additional safety and pharmacokinetic studies are warranted owing to the role of SDH inhibition in multiple cancers, which will pave the way to harnessing the regenerative effect of malonate resulting from its simplicity and efficacy. Malonate provides an opportunity for transient SDH inhibition, but the long-term effects also need to be determined. Promoting adult heart regeneration by SDH inhibition has enormous implications for the treatment of patients with systolic heart failure.
Malonate is found in many fruits and vegetables
Malonic acid is found in small amounts in sugar beet and green wheat, being formed by oxidative degradation of malic acid. Malonic acid has been identified in celery, parsnip, green pepper, tangerine, beet, oats, tabasco, chili, orange, grapefruit, lime, melon, cantaloupe, sunflower, barley, prickly pear, Indian fig, ginseng, black bean, kidney bean, green bean, peach, corn, Scotch pine, alfalfa, banana, tobacco plant, and juniper.Malonic acid
Malonic acid | C3H4O4 | CID 867 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
Linoleate reduces succinate dehydrogenase activity
When a ground squirrel wants to hibernate, it eats acorns. Acorns are loaded with highly unsaturated fat – most of it is monounsaturated and 20% or so is polyunsaturated (PUFA). Squirrels who don’t eat enough PUFA fail to enter torpor. Each unsaturated bond is one less FADH2 input and lowers SDH activity. Low SDH activity means minimal ROS generation.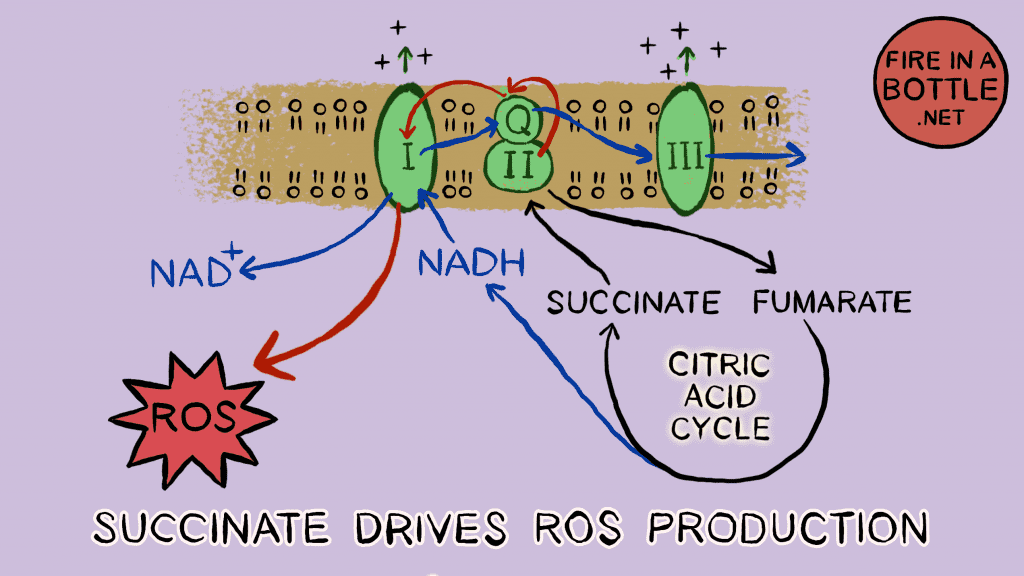
Obesity as a Global Succinate Dehydrogenase Activity Deficit - The ROS Theory of Obesity Part III, Part 2 - Fire In A Bottle
I’m using the word global to mean “in all tissues of the body” but I’d forgive you if you thought that I meant “in all countries where they eat vegetable oil”. Either way, really. As I explained in part 1, the production of mitochondrial ROS is a thermogenic loop that wastes calories as heat...
 fireinabottle.net
fireinabottle.net
Last edited:
