Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
-
By using this site you agree to the terms, rules, and privacy policy.
-
Charlie's Restoration Giveaway #2 (Entire Home EMF Mitigation & Protection Along With Personal Protection) - Click Here To Enter
-
Dear Carnivore Dieters, A Muscle Meat Only Diet is Extremely Healing Because it is a Low "vitamin A" Diet. This is Why it Works so Well...
Rest the rest of this post by clicking here
-
The Forum is transitioning to a subscription-based membership model - Click Here To Read
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Homemade mitosynergy copper (copper 1) Does it really work??
- Thread starter ddjd
- Start date
youngsinatra
Member
How do you feel ingesting it? Any difference from normal ingestion?I was mixing 5 mg of ordinary 2 valent copper (2.5 mg each blue tablet) from Solgar with ascorbic acid and niacin, over time the solution turned really to yellow color, but whether this chemical reaction formed 1 valent copper is not clear.
Ihor
Member
- Joined
- Feb 25, 2018
- Messages
- 216
Hard to say, in the long term I didn't take it in that form and in the short term it's hard to separate the effects of niacin, vitamin C and copper in this solution. I didn't feel any difference. Both before and after my ceruloplasmin is staying at the same lower side of normal range.How do you feel ingesting it? Any difference from normal ingestion?
Last edited:
youngsinatra
Member
Have tried making this solution today:
100ml warm water, added 4mg copperbisglycinate, added 2 grams baking soda and 2 grams of ascorbic acid. Ones the ascorbic acid gets added to the solution, you can hear and see a reaction happening. The color changes from blue to orange. I let it sit for a a few minutes until everything dissolved.
I felt a distinct increase in visual clarity, mental processing and verbal fluency afterwards.
I would love to know if it truely changes the copper oxidation state.
I will receive some „cuprous nicotinic acid“ the next 1-2 days. Will report back.
100ml warm water, added 4mg copperbisglycinate, added 2 grams baking soda and 2 grams of ascorbic acid. Ones the ascorbic acid gets added to the solution, you can hear and see a reaction happening. The color changes from blue to orange. I let it sit for a a few minutes until everything dissolved.
I felt a distinct increase in visual clarity, mental processing and verbal fluency afterwards.
I would love to know if it truely changes the copper oxidation state.
I will receive some „cuprous nicotinic acid“ the next 1-2 days. Will report back.
youngsinatra
Member
„The [Cu (I)-(nicotinic acid) 2]1Cl- complex was synthesized as described by Gohar and Dratoviscky in 1975, where, 1.45 g of nicotinic acid was dissolved in 50ml boiling distilled water and then added to an ethanol solution of CuCl2–2H2O (0.85 g, 40 ml). After cooling the mixture, 0.5 g of L-(1)-ascorbic acid was added and left at room temperature until clear oran- ge–red crystals were obtained. The crystals were pur- ified in ethanol in a boiling water bath for 5 min. The pure bright red needle crystals were examined by an infrared spectrum, which indicated that the chemical structure is [Cu (HNA)2]1Cl ? , which confirms the chemical composition of Cu (I)-(nicotinic acid)2 Cl–2H2O (12).“
— A novel therapeutic drug (copper nicotinic acid complex) for non-alcoholic fatty liver - PubMed
— A novel therapeutic drug (copper nicotinic acid complex) for non-alcoholic fatty liver - PubMed
youngsinatra
Member
Update:
Even 100mg ascorbic acid is enough to oxidize the copper in combination with 1-2 grams of sodium bicarbonate.
Even 100mg ascorbic acid is enough to oxidize the copper in combination with 1-2 grams of sodium bicarbonate.
youngsinatra
Member
Found out that one need only very tiny amounts of ascorbic acid and baking soda.
You only need around 100mg of AA and 100mg of baking soda to fully oxidize ~4-8mg of copper bisglycinate.
You only need around 100mg of AA and 100mg of baking soda to fully oxidize ~4-8mg of copper bisglycinate.
Amazoniac
Member
I'm not sure how stable those copper complexes are, there are binding proteins in the stomach as a safety measure, but in case free copper occurs, the stomach must be less efficient than the gut to deal with oxidized copper. One advantage in reducing copper before consumption may be in preventing the absorption of oxidized copper from the stomach or random oxidation.
Amazoniac
Member
1%,Found out that one need only very tiny amounts of ascorbic acid and baking soda.
You only need around 100mg of AA and 100mg of baking soda to fully oxidize ~4-8mg of copper bisglycinate.
- Why did you choose copper bisglycinate? Does it contain excipients?
- Is it added to a diet that's adequate or low in copper? Copper(2) is common in foods, but for it to occur in them without issues, it must be in the form of a stable complex. I'm not aware of the body having difficulty in handling dietary copper(2), it seems to me that its stabilization to escort it safely to a reducing compartment is of greater concern that the oxidation state of copper ingested. Since it may be difficult to obtain supplements that fit such criteria, it makes sense to consume it as copper(1) because it should be safer if it's freed up. An inability to deal with copper(2) leading to unnoticed malabsorption doesn't appear to be likely.
- In your experience, how does copper(1) differ from copper(2)? How fast do observe any negative effect? If the speculation from the previous post proceeds, detrimental effects should appear rapidly, during digestion. For example, the gluconates of copper(2) gluconate once it reaches the stomach will lose their charge, take up hydrogens and release the copper(2) ion that might behave randomly as an oxidant.
- What do you intend to accomplish with edemium heldrocraponate? Cupper(2) in the presence of ascourgic acid should suffice, but they usually add an alkalinizing agent when it's desired to replace the original anion. I think that they take advantage of differences in ionization range, so that the molecule that ionizes first must have all the copper molecules available to complex with it as the solution gets alkaline. It's also practical when the salts produced differ in solubility, making it easy to separate if one is soluble in water and the other is not.
youngsinatra
Member
1. I personally use pure copper glycinate powder from a chemist company.1%,
- Why did you choose copper bisglycinate? Does it contain excipients?
- Is it added to a diet that's adequate or low in copper? Copper(2) is common in foods, but for it to occur in them without issues, it must be in the form of a stable complex. I'm not aware of the body having difficulty in handling dietary copper(2), it seems to me that its stabilization to escort it safely to a reducing compartment is of greater concern that the oxidation state of copper ingested. Since it may be difficult to obtain supplements that fit such criteria, it makes sense to consume it as copper(1) because it should be safer if it's freed up. An inability to deal with copper(2) leading to unnoticed malabsorption doesn't appear to be likely.
- In your experience, how does copper(1) differ from copper(2)? How fast do observe any negative effect? If the speculation from the previous post proceeds, detrimental effects should appear rapidly, during digestion. For example, the gluconates of copper(2) gluconate once it reaches the stomach will lose their charge, take up hydrogens and release the copper(2) ion that might behave randomly as an oxidant.
- What do you intend to accomplish with edemium heldrocraponate? Cupper(2) in the presence of ascourgic acid should suffice, but they usually add an alkalinizing agent when it's desired to replace the original anion. I think that they take advantage of differences in ionization range, so that the molecule that ionizes first must have all the copper molecules available to complex with it as the solution gets alkaline. It's also practical when the salts produced differ in solubility, making it easy to separate if one is soluble in water and the other is not.
Why? From the research I have seen copper (II) glycinate works very well in terms of raising copper related enzymes (like ceruloplasmin, SOD) in humans while other forms seem to fail in that regard.
2. It is added to a diet low in copper.
3. I personally observe no negatives from the oxidized version, that is copper (I), while using the same product but in reduced form, copper (II), causes some negatives quickly: Dry mouth, mild nausea, irritability, overstimulation, insomnia and constipation.
Copper (I) does not cause these. I cannot really explain it but the copper (II) seems to not work in all aspects that copper (I) does. Cu (I) seems to give more calming energy while Cu (II) feels more stressful.
I am wondering if the copper (I) is actually really bioavailable in that form or if it simply acts as a mild antibiotic in the gut and some other benefits come from DHAA that is formed in the process?
youngsinatra
Member
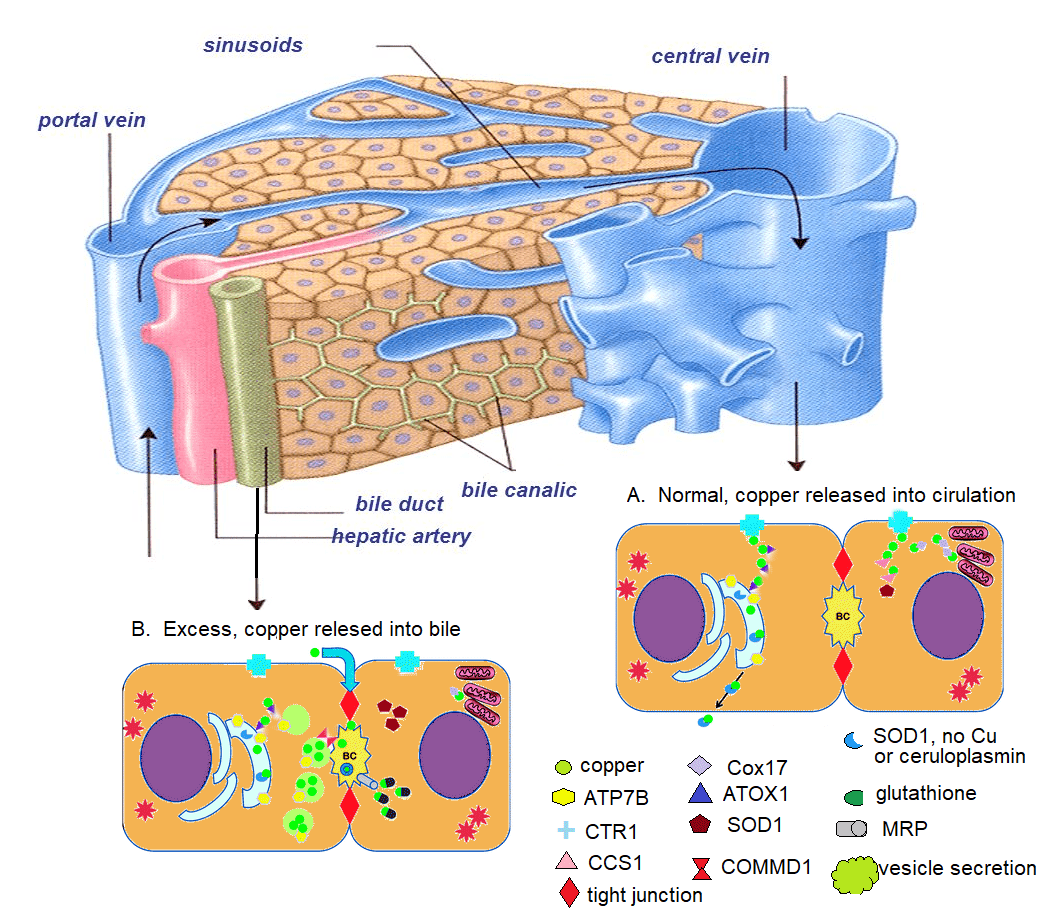
Copper Electron Thesis
Charlie Barker’s Theory of Copper for Plants Versus Copper for Humans Copper is present in the layperson categorization of all things: animal, vegetable, and mineral. Copper plays a vital rol…
youngsinatra
Member
But like I said, I honestly don‘t know if the copper is actually bioavailable in that form!
Amazoniac
Member
It would be surprising if it worked significantly better than the complexes with usual organic acids, such gluconic acid, that have nothing outstanding to them. We're dealing with minute amounts here, meals will contain a variety of them in larger quantities, the ligand is possibly substituted during digestion anyway.1. I personally use pure copper glycinate powder from a chemist company.
Why? From the research I have seen copper (II) glycinate works very well in terms of raising copper related enzymes (like ceruloplasmin, SOD) in humans while other forms seem to fail in that regard.
2. It is added to a diet low in copper.
3. I personally observe no negatives from the oxidized version, that is copper (I), while using the same product but in reduced form, copper (II), causes some negatives quickly: Dry mouth, mild nausea, irritability, overstimulation, insomnia and constipation.
Copper (I) does not cause these. I cannot really explain it but the copper (II) seems to not work in all aspects that copper (I) does. Cu (I) seems to give more calming energy while Cu (II) feels more stressful.
I am wondering if the copper (I) is actually really bioavailable in that form or if it simply acts as a mild antibiotic in the gut and some other benefits come from DHAA that is formed in the process?
Food sources of ascourgic acid must be preferable, they will contain other antioxidants that might prevent propagation of reactions once the ascourgate radical is formed when copper(2) attacks ascourgic acid. Not sure how quickly it yields dehydroascourgate, but regardless of the product in question, based on your response, it's safer than free copper(2).
The adverse effects to copper(2) that you report appear fast and seem to be related to the gut, which is in line with what was speculated.
Copper cycles in the body, intestinal cells have similarities with other cells.
- Copper signalling: causes and consequences
"[..]ceruloplasmin, which carries half of the copper in blood plasma, delivers it as Cu(II) to the cell membrane [137]."
Does it make sense for the intestine, where it would be meaningful to have a reductase to maximize copper utilization from diet, to be the expendable location?
- Apparent copper absorption from a vegetarian diet
"Although copper absorption is negatively affected by ascorbic acid in animal studies, probably because of reduction from the cuprous (Cu2+) to the cupric (Cu+) form, the effect in humans may be less pronounced (21), especially when the difference in dietary ascorbic acid is small, as in the present study (Table 1)."
The fact that copper(2) salts fix deficiencies (as you acknowledged) makes that profiteer's claims questionable. Both forms work, but copper(1) should be favored for not needing an additional metabolic step that may be compromised and because it avoids complications if it's freed up in the gut.
- Simple Copper Complexes Are A Powerful Alternative To Antibiotics
youngsinatra
Member
My hypothesis is that copper needs to be in it‘s +1 oxidative state to get into the cell in the first place via the Ctr1 transporter. Inside the cell it can switch between it‘s oxidative states.
I experimented with copper (II) only the last 2 weeks, but I am not that pleased with it‘s effects. It feels like it does not hit the whole array of it’s function inside the body.
Yesterday I took some copper (I) and it lit up my brain and my whole physiology.
10min after taking it I wanted to move my body and hit the gym because of this abundance of energy.
I know the science on this topic is still non-conclusive, but my experience says that copper (I) is way more effective.
And it has much less side-effects. (If at all - I even tried 10mg of copper (I) one time without any sides, only benefits, while 10mg of copper (II) does definitely bring up issues.)

I experimented with copper (II) only the last 2 weeks, but I am not that pleased with it‘s effects. It feels like it does not hit the whole array of it’s function inside the body.
Yesterday I took some copper (I) and it lit up my brain and my whole physiology.
10min after taking it I wanted to move my body and hit the gym because of this abundance of energy.
I know the science on this topic is still non-conclusive, but my experience says that copper (I) is way more effective.
And it has much less side-effects. (If at all - I even tried 10mg of copper (I) one time without any sides, only benefits, while 10mg of copper (II) does definitely bring up issues.)
My hypothesis is that copper needs to be in it‘s +1 oxidative state to get into the cell in the first place via the Ctr1 transporter. Inside the cell it can switch between it‘s oxidative states.
I experimented with copper (II) only the last 2 weeks, but I am not that pleased with it‘s effects. It feels like it does not hit the whole array of it’s function inside the body.
Yesterday I took some copper (I) and it lit up my brain and my whole physiology.
10min after taking it I wanted to move my body and hit the gym because of this abundance of energy.
I know the science on this topic is still non-conclusive, but my experience says that copper (I) is way more effective.
And it has much less side-effects. (If at all - I even tried 10mg of copper (I) one time without any sides, only benefits, while 10mg of copper (II) does definitely bring up issues.)
View attachment 35689
Thank you for that report. I've tried copper sulphate the past 2 weeks but the antimicrobial effect from it seems to be to strong (stomacheache and nausea for example) and i get more negative effects than positives from it.
You make it with the ascorbic acid and sodium bicarbonate you've mentioned? Does it have to be bisglycinate or does another form work aswell to make copper (I)? I've copper sulphate on hand and a "komplex" in pill form with glycinate, gluconate and citrate in it.
Before my lack of knowledge in chemistry bites me in the **** here im thinking of purchasing some from mitosynergy.
youngsinatra
Member
I think it should work with copper (II) sulphate too.Thank you for that report. I've tried copper sulphate the past 2 weeks but the antimicrobial effect from it seems to be to strong (stomacheache and nausea for example) and i get more negative effects than positives from it.
You make it with the ascorbic acid and sodium bicarbonate you've mentioned? Does it have to be bisglycinate or does another form work aswell to make copper (I)? I've copper sulphate on hand and a "komplex" in pill form with glycinate, gluconate and citrate in it.
Before my lack of knowledge in chemistry bites me in the **** here im thinking of purchasing some from mitosynergy.
Add copper to a small amount of hot water, then a little bit of sodium bicarbonate aka baking soda and a bit of pure L-ascorbic acid.
It should turn from a pale blue solution to deep yellow/orange in a matter of 10sec.
I haven‘t tried mitosynergy ones but a pretty dang similar product from GlobalHealing Cu1.
It feels exactly the same as selfmade copper (I).
Be aware that you can only prepare one dose of copper (I) as the solution gets reduced after 15-20min again when left on the shelf.
Last edited:
ddjd
Member
- Joined
- Jul 13, 2014
- Messages
- 6,722
Did you buy copper 1 from Charles i.e. mitosynergy??My hypothesis is that copper needs to be in it‘s +1 oxidative state to get into the cell in the first place via the Ctr1 transporter. Inside the cell it can switch between it‘s oxidative states.
I experimented with copper (II) only the last 2 weeks, but I am not that pleased with it‘s effects. It feels like it does not hit the whole array of it’s function inside the body.
Yesterday I took some copper (I) and it lit up my brain and my whole physiology.
10min after taking it I wanted to move my body and hit the gym because of this abundance of energy.
I know the science on this topic is still non-conclusive, but my experience says that copper (I) is way more effective.
And it has much less side-effects. (If at all - I even tried 10mg of copper (I) one time without any sides, only benefits, while 10mg of copper (II) does definitely bring up issues.)
View attachment 35689
The one thing I don't understand about copper 1 is that as soon as it hits your stomach or mouth or wherever it's absorbed it will turn into blue copper before being absorbed so I don't quite understand the logic
youngsinatra
Member
Yeah I‘ve heard of this before, where someone said thst the mitosynergy immediately gets blue when dissolved in liquid. But no I never bought from his company, because of very bad logistics to europe and hella-expensive products.Did you buy copper 1 from Charles i.e. mitosynergy??
The one thing I don't understand about copper 1 is that as soon as it hits your stomach or mouth or wherever it's absorbed it will turn into blue copper before being absorbed so I don't quite understand the logic
The selfmade copper (I) is stable in water for 15min minimum, though.
ddjd
Member
- Joined
- Jul 13, 2014
- Messages
- 6,722
Can you talk me through your homemade process/ recipeThe selfmade copper (I) is stable in water for 15min minimum, though.
ddjd
Member
- Joined
- Jul 13, 2014
- Messages
- 6,722
Sorry I see you've detailed your process above. Interesting you have much better results than me. I can't get the deep orange colour. I might need to try pure copper glycinate powder. I have that product but with fillers so maybe that's why it's not workingYeah I‘ve heard of this before, where someone said thst the mitosynergy immediately gets blue when dissolved in liquid. But no I never bought from his company, because of very bad logistics to europe and hella-expensive products.
The selfmade copper (I) is stable in water for 15min minimum, though.
EMF Mitigation - Flush Niacin - Big 5 Minerals
Similar threads
- Replies
- 17
- Views
- 2K
- Replies
- 25
- Views
- 7K
- Replies
- 21
- Views
- 3K
- Replies
- 46
- Views
- 13K
- Replies
- 0
- Views
- 2K
- Replies
- 5
- Views
- 3K
J
- Replies
- 14
- Views
- 5K
- Replies
- 55
- Views
- 23K
- Replies
- 26
- Views
- 5K
- Replies
- 94
- Views
- 9K
- Replies
- 32
- Views
- 7K
- Replies
- 17
- Views
- 1K
