Yes, I’m pretty fair skin of northwestern European ancestry and there isn’t much sun here. Skin tone is definitely a factor. This is the first year I haven’t needed D in winter but I was doing 10k and getting lots of sunshine last year so I probably have good stores. Some people are pro D and some anti D but I think it’s highly individual. I’m grateful to have had access to D supplements when I needed them.Oh thanks for the info. I suppose I don't need UV because I live already in a sunny country. But I don't think I get enough vitamin D from our sun because I wear the white traditional clothes, so I am almost covered from head to toe and I am naturally a tanned person.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
-
By using this site you agree to the terms, rules, and privacy policy.
-
Charlie's Restoration Giveaway #2 (Entire Home EMF Mitigation & Protection Along With Personal Protection) - Click Here To Enter
-
Dear Carnivore Dieters, A Muscle Meat Only Diet is Extremely Healing Because it is a Low "vitamin A" Diet. This is Why it Works so Well...
Rest the rest of this post by clicking here
-
The Forum is transitioning to a subscription-based membership model - Click Here To Read
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Low Toxin Diet Grant Genereux's Theory Of Vitamin A Toxicity
SaltGirl
Member
- Joined
- Oct 18, 2013
- Messages
- 178
Just wanted to chime in on the Vitamin A issue. I do not want to advocate for either camp as I am a bit unsure myself, but I have found my health often deteriorating when I am eating higher Vitamin A. Worst case when I was supplementing concentrated Vitamin A which - if I remember correctly - had about 10.000 IU each in every pill. This was at the time when the forum and others were high on Vitamin A and thought it would be the next big hit.
A lot of stuff coincided with that time and for all I know these things could be concidences, but for the sake of posterity I am going to list a few:
- One molar broke almost out of nowhere.
- Dental health deteriorated rapidly.
- Vision in one eye became super bad. After a year of much less Vitamin A than I ate before the eye has been slowly fixing itself. Still not as good as it was.
- Blood pressure increased considerably.
- Other smaller issues, but these were the ones I noticed and remembered.
Again, these things might just be coincidences, but I have been thinking a lot lately how this coincided with my Vitamin A time.
Looking over my life I must admit that I have never had an affinity for Vitamin A rich foods bar perhaps milk and cheese. Hate liver, hate more or less every vegetable high in Vitamin A(Red Bell pepper in certain contexts being the only odd thing out). I do not like fruits high in Vitamin A, and organ meat I just hate and I grew up in a homestead that had organ meat every single week that I avoided like the plague.
So I admit that I have found this thread to be very interesting. I have some theories of my own, but nothing concrete.
Another interesting sidenote is that my Vitamin C needs increased substantially after my Vitamin A period.
A lot of stuff coincided with that time and for all I know these things could be concidences, but for the sake of posterity I am going to list a few:
- One molar broke almost out of nowhere.
- Dental health deteriorated rapidly.
- Vision in one eye became super bad. After a year of much less Vitamin A than I ate before the eye has been slowly fixing itself. Still not as good as it was.
- Blood pressure increased considerably.
- Other smaller issues, but these were the ones I noticed and remembered.
Again, these things might just be coincidences, but I have been thinking a lot lately how this coincided with my Vitamin A time.
Looking over my life I must admit that I have never had an affinity for Vitamin A rich foods bar perhaps milk and cheese. Hate liver, hate more or less every vegetable high in Vitamin A(Red Bell pepper in certain contexts being the only odd thing out). I do not like fruits high in Vitamin A, and organ meat I just hate and I grew up in a homestead that had organ meat every single week that I avoided like the plague.
So I admit that I have found this thread to be very interesting. I have some theories of my own, but nothing concrete.
Another interesting sidenote is that my Vitamin C needs increased substantially after my Vitamin A period.
milk_lover
Member
- Joined
- Aug 15, 2015
- Messages
- 1,909
I am on the fence regarding vitamin D. I react poorly to vitamin E. I haven't tried the new tocovit formula to see if vitamin E is the problem or the quality of product. Vitamin K2 seems to be the only fat soluble vitamin that I can tolerate easily. So my instinct is telling me that maybe we only truly need to supplement vitamin K2 and occasionally vitamin E. A and D can come from food and the sun.Yes, I’m pretty fair skin of northwestern European ancestry and there isn’t much sun here. Skin tone is definitely a factor. This is the first year I haven’t needed D in winter but I was doing 10k and getting lots of sunshine last year so I probably have good stores. Some people are pro D and some anti D but I think it’s highly individual. I’m grateful to have had access to D supplements when I needed them.
Thanks @SaltGirl. I’ve noticed some of the same things when looking back on my own life.Just wanted to chime in on the Vitamin A issue. I do not want to advocate for either camp as I am a bit unsure myself, but I have found my health often deteriorating when I am eating higher Vitamin A. Worst case when I was supplementing concentrated Vitamin A which - if I remember correctly - had about 10.000 IU each in every pill. This was at the time when the forum and others were high on Vitamin A and thought it would be the next big hit.
A lot of stuff coincided with that time and for all I know these things could be concidences, but for the sake of posterity I am going to list a few:
- One molar broke almost out of nowhere.
- Dental health deteriorated rapidly.
- Vision in one eye became super bad. After a year of much less Vitamin A than I ate before the eye has been slowly fixing itself. Still not as good as it was.
- Blood pressure increased considerably.
- Other smaller issues, but these were the ones I noticed and remembered.
Again, these things might just be coincidences, but I have been thinking a lot lately how this coincided with my Vitamin A time.
Looking over my life I must admit that I have never had an affinity for Vitamin A rich foods bar perhaps milk and cheese. Hate liver, hate more or less every vegetable high in Vitamin A(Red Bell pepper in certain contexts being the only odd thing out). I do not like fruits high in Vitamin A, and organ meat I just hate and I grew up in a homestead that had organ meat every single week that I avoided like the plague.
So I admit that I have found this thread to be very interesting. I have some theories of my own, but nothing concrete.
Another interesting sidenote is that my Vitamin C needs increased substantially after my Vitamin A period.
milk_lover
Member
- Joined
- Aug 15, 2015
- Messages
- 1,909
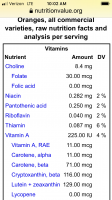
Honestly, lemonade and grape juices taste much better for me than OJ. I may order some from the cafeteria tonight before Real Madrid Barcelona game.View attachment 12361
I’m just not including right now because of the carotenoids and I’m drinking lemonade, grape and apple juice instead.
Enjoy the game and your juice!Honestly, lemonade and grape juices taste much better for me than OJ. I may order some from the cafeteria tonight before Real Madrid Barcelona game.
milk_lover
Member
- Joined
- Aug 15, 2015
- Messages
- 1,909
Thanks! I know I will be stressed watching the game, so I better drink some sugars to bring down cortisol levels.Enjoy the game and your juice!
Amazoniac
Member
- Glossary of Immumology terms | Immunopaedia
- Retinoic Acid and Retinoic Acid Receptors as Pleiotropic Modulators of the Immune System
- Retinoic Acid and Immunity (same boss)
- Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells (check out figures)
- Retinoic Acid and Its Role in Modulating Intestinal Innate Immunity
- Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases
- Retinoic Acid, Leaky Gut, and Autoimmune Diseases (@Tarmander - 3.1/4.3.1)
- Retinoic acid syndrome: a review (massive amounts given in cancre, perhaps there's something to learn from it)
- Vitamin A-Deficient Hosts Become Nonsymptomatic Reservoirs of Escherichia coli-Like Enteric Infections
--
Complementing post #1693..
- Inflamed About Retinoic Acid
↳ Context is key in the gut (don't be fooled by the title)
- Reciprocal TH17 and Regulatory T Cell Differentiation Mediated by Retinoic Acid
- Paradoxical Effects of All-Trans-Retinoic Acid on Lupus-Like Disease in the MRL/lpr Mouse Model
--
Propoison A:
- Concentrations of Selected Carotenoids and Vitamin A in Human Liver, Kidney and Lung Tissue
- Mammalian carotenoid absorption and metabolism
- Carotenoids: Linking Chemistry, Absorption, and Metabolism to Potential Roles in Human Health and Disease
- Evidence for compartmentalization of mammalian carotenoid metabolism
- β-Carotene conversion products and their effects on adipose tissue
- A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries)
- Presence of fed β-carotene in digesta, excreta, blood, andhepatic and adipose tissues of Holstein steers
- Carotenoid trade-off between parasitic resistance and sexual display: an experimental study in the blackbird (Turdus merula) (it's cool)
I'm counting on you US and A citizens to start a chain reaction by requesting from your local producers 'The Return of White Carrots'. They should be featured on your Instagram pages as part of artificial dishes, which in turn will influence artificial people from unimportant countries to copy what you're doing, which in turn will lead to improvised pictures using radishes but at the same time create multiple local demands for producers to grow more of the real deal, and we'll conclude the reactions with a comment with hints of hypotyphoid selfishness that it will eventually benefit me for making it easier to buy them. Thank you as well in advance.
- Retinoic Acid and Retinoic Acid Receptors as Pleiotropic Modulators of the Immune System
"Although the all-trans RA (atRA) isoform predominates in most tissues, additional isoforms of RA can be detected, such as 13-cis-RA. The specific effects of RA are critically dose dependent and therefore depend on the spatial and temporal regulation of RA metabolism, transportation, and diffusion, which determine the intracellular and tissue-specific concentration gradients."
"A variant of atRA, 9-cis-RA, is equally capable in activating RARs, but atRA is 50-fold weaker in activating RXRs (15). The biological significance of 9-cis-RA as an RXR ligand remains controversial because 9-cis-RA is hardly detectable in vivo (16). Furthermore, oleic acid and docosahexaenoic acid also bind and activate RXR (9), and given that RXRs also associate with other nuclear receptors that participate in fatty acid biosynthesis, metabolism, or transport, it is conceivable that the true natural ligands of RXR are fatty acids (17). Nevertheless, the activation of RXR as part of the RAR/RXR complex is silenced in the absence of RAR ligands, a phenomenon referred to as RXR subordination. The participation of RXR is mainly to enhance the DNA binding of the heterodimer, and simultaneous ligation of the receptors augments the transcriptional activation of RARs (18)."
"In general, retinoid receptors reside in the nucleus; however, in some cells or under certain conditions, they can be found in the cytoplasm, where they display nongenomic functions in a ligand-dependent or -independent fashion and as monomers or complexed with various cellular factors (14) []. The ability of these receptors to participate in extranuclear signaling cascades adds to the complexity and pleiotropic effects of their ligand-dependent and -independent roles."
"In addition to RARs, RXRs can form heterodimeric complexes with other nuclear receptors, such as the thyroid hormone receptor, the vitamin D3 receptor, the COUP-TFII, and the peroxisome proliferator–activated receptors (PPARs). Like RARs, the PPAR subfamily consists of several subtypes. In general, PPARs are lipid sensors activated by fatty acids. However, the PPARβ/δ isotype displays significant affinity for atRA as well; therefore, RA may activate transcription not only through RARs but also through PPARβ/δ (20). In contrast to CRABPII, which transports RA to the nucleus and greatly facilitates RA-RAR ligation and transcriptional activation of RAR-dependent target genes, PPARs interact with fatty acid–binding proteins (FABPs), and in the case of FABP5, the RA-FABP5 complex engages and activates nuclear PPARβ/δ. Therefore, depending on the presence of CRABPII or FABP5, RA can activate either nuclear RARs or PPARβ/δ. Because the binding affinity of CRABPII/RAR for RA is much stronger than for FABP5/PPARβ/δ, RAR-mediated transcriptional activity is dominant. However, higher expression of FABP5 or absence of CRABPII will result in the activation of PPARβ/δ gene transcription in the presence of RA. Given that RA-ligated PPARβ/δ complexes control characteristically antiapoptotic genes, they could overcome the growth-inhibitory activities of RARs and drive survival in the presence of proapoptotic agents (21). Cyclin D3 can also interact with CRABPII in a ligand-independent way, and as a nuclear complex it will enhance the transcriptional activity of RA-ligated RARs (22)."
"The activity of the inflammatory associated transcription factor NF-κB can be repressed by RA []; consequently, NF-κB activation (30) and lipopolysaccharide (LPS)-mediated inflammatory responses (31) are much enhanced under VAD conditions."
"The immune system is greatly influenced by a broad range of effects mediated by retinoids, not only for the development of primary and secondary lymphoid tissues but also for the functional differentiation of various immune cells and the control of inflammatory as well as tolerogenic immune responses. The specific effects RA and its receptors have on the immune response are not always predictable, and depending on the dose, the RAR isoform, the cell type, or the environmental conditions, the outcome can be very different and sometimes paradoxical, suppressing the development of one cell type but promoting at the same time the development of another type or driving immunosuppressive effects in one situation but promoting inflammatory responses in another context. The fact that RA as an exogenous factor directly communicates with nuclear receptors inside the cell, thereby controlling cell fate, provides the immune system with a powerful rheostat to sense the environment and not only control development but also fine-tune the immune response in the most effective and most compatible way to warrant optimal protection with the least damage to the body."
"Retinoids play critical roles during development and differentiation of primary lymphoid organs (44). The thymus, which during embryonic development originates from a pouch of the foregut endodermal tube, may have evolved originally from the primordial gut-associated lymphoid tissue (GALT), which formed the primary lymphoid organ before the appearance of a thymus. As in the intestinal epithelium, RA and RARs are important regulators of thymic epithelial cell homeostasis, and thymic mesenchymal cells are a major source of RA during embryogenesis (45). Furthermore, in developing thymocytes, ligation of RARγ/RXR heterodimers by atRA or 9-cis- RA leads to glucocorticoid-mediated cell death involved in apoptosis by neglect of double-positive (DP) thymocytes. Hence, in vivo treatment with RARγ agonists results in thymic involution due to massive death of DP thymocytes (46, 47). Activation of RARα by large doses of atRA can override RARγ-induced death, whereas strong costimulation of RXR by 9-cis-RA neutralizes the inhibitory effect of RARα (46)."
"Although critical for embryonic development and lymphopoiesis, RA and RARs are first and foremost effective at controlling postnatal immune functions, especially at the mucosal border of the intestine. Flexibility to balance between optimal protective immunity and effective peripheral tolerance is a prerequisite for the mucosal immune system that operates at this largest interface of the body and is thus exposed to numerous pathogen-derived antigens but at the same time is continuously exposed to a vast load of antigens from the diet and commensals that need to be tolerated. Aberrant immune responses directed towards such innocuous antigens can cause local chronic inflammation that often spreads systemically. Consequently, optimal protective immunity has to coincide with effective immune regulation, and RA and its receptors play critical roles in maintaining this balance. Although RA is typically associated with immunosuppressive roles, RA and RARs can also function as initiators of inflammatory responses and protective immunity. Because of this flexibility, these molecules are ideal to control mucosal immune responses and immunity in general; therefore, just about every immune cell is profoundly affected in one way or the other by the signals and molecular mechanisms controlled by RA and its receptors."
"Besides inducing RALDH2 expression, RA influences the maturation and antigen-presentation function of DCs and their ability to prime T cells in either a tolerogenic or an inflammatory fashion."
"Paradoxically, although [] RA-based regulatory functions are all critical to maintain immune homeostasis and warrant steady state immune tolerance, RA also mediates proinflammatory effects on DCs and promotes proinflammatory priming of T cells during an infection or under inflammatory conditions. Furthermore, instead of promoting apoptosis of antigen-presenting DCs, RAR/RXR signaling in conjunction with inflammatory stimuli can lead to survival and maturation of inflammatory DCs, which then upregulate their production of RA in a feedback-loop system and promote increased RA signaling at sites of inflammation (72). RA can further cooperate with inflammatory cytokines such as IL-15 to enhance the production of IL-12p70 and IL-23 by DCs (73) (Figure 3)."

"The effect of RA on macrophages, similar to its effect on DCs, can be anti- or proinflammatory. Upon LPS stimulation, for example, RA decreases the production of IL-12, TNF-α, nitric oxide (NO), PGE2, or COX2 (75–78), whereas it enhances the production of IL-10 (77). Furthermore, IL-10 can feed back on the macrophages and further limit their production of proinflammatory cytokines (79). Alternatively, RA can cooperate with GM-CSF to promote the phagocytic antigen presentation function of macrophages leading to induced regulatory T cell (Treg) formation and immune tolerance (65). In contrast, ligation of RARγ in macrophages controls optimal production of inflammatory cytokines in response to microbial stimuli resulting in a more effective pathogen control, as observed in a Mycobacterium tuberculosis infection (80). Overall, these observations indicate that RA and RAR signaling in mucosal APCs greatly affects the course of the immuneresponse, underscoring the central role of RA in controlling the balance between mucosal immunetolerance and protective immunity."
"The effects of RA signaling in T cells, similar to their effects on the APCs, are context dependent and can be promoting or inhibiting depending on the local and/or intracellular dose of RA, the expression of specific RAR isoforms, the specific cytokine environment, and the nature of the responding T cell."
"Despite its role in the reciprocal regulation of enhanced iTreg generation but suppressed Th17 cell differentiation, RA at low doses is also essential for the functional differentiation of effector T cells. In addition to impaired homing to the site of inflammation, differentiation of effector CD4 T cells is also defective in VAD mice or mice deficient for RARα (43). Furthermore, IFN-γ and IL-17A production by Th1 and Th17 cells in response to an infection was reduced in the absence of RA signaling in T cells (43). Similar results were observed in mouse models of vaccination or allogeneic graft rejection when transcriptional activity of RARs was specifically ablated in CD4 T cells (106). Although linked to the pathogenesis of autoimmune and excessive inflammatory responses, Th17 cells are equally important for protective immunity at the mucosal interface of the intestine, and they are typically present at steady state in this compartment, but they are virtually ablated in VAD mice (43). The ability of APCs to produce IL-6 is also reduced in VAD mice (43), and in a proinflammatory context involving cytokines, such as IL-15, RA acts through DCs to block conversion into Tregs and to promote Th1 effector polarization (73). Therefore, the ability of RA to support effector differentiation probably results from combined actions on DCs and T cells, because addition of RA to Th17 cell–polarizing conditions in APC-less cultures did not enhance effector polarization (107)."
"[..]although mucus plays a critical role in protecting the intestinal barrier, vitamin A has a negative effect on mucus production by goblet cells, which conversely increased under VAD conditions (160). [¿] Enhanced mucus production is further promoted by IL-13 produced by the expanded ILC2 population in the absence of vitamin A (160). Therefore, although an insufficiency of vitamin A greatly impairs the adaptive Th2 response, it equally increases the passive barrier protection and the innate ILC2 response and thus maintains or even increases control of nematode infections (160) (Figure 3). The balanced compensation of RA/RAR signaling in the innate and adaptive immune system allow for flexibility and warrant effective protective immunity adjustable to the circumstances or environmental triggers."
"A variant of atRA, 9-cis-RA, is equally capable in activating RARs, but atRA is 50-fold weaker in activating RXRs (15). The biological significance of 9-cis-RA as an RXR ligand remains controversial because 9-cis-RA is hardly detectable in vivo (16). Furthermore, oleic acid and docosahexaenoic acid also bind and activate RXR (9), and given that RXRs also associate with other nuclear receptors that participate in fatty acid biosynthesis, metabolism, or transport, it is conceivable that the true natural ligands of RXR are fatty acids (17). Nevertheless, the activation of RXR as part of the RAR/RXR complex is silenced in the absence of RAR ligands, a phenomenon referred to as RXR subordination. The participation of RXR is mainly to enhance the DNA binding of the heterodimer, and simultaneous ligation of the receptors augments the transcriptional activation of RARs (18)."
"In general, retinoid receptors reside in the nucleus; however, in some cells or under certain conditions, they can be found in the cytoplasm, where they display nongenomic functions in a ligand-dependent or -independent fashion and as monomers or complexed with various cellular factors (14) []. The ability of these receptors to participate in extranuclear signaling cascades adds to the complexity and pleiotropic effects of their ligand-dependent and -independent roles."
"In addition to RARs, RXRs can form heterodimeric complexes with other nuclear receptors, such as the thyroid hormone receptor, the vitamin D3 receptor, the COUP-TFII, and the peroxisome proliferator–activated receptors (PPARs). Like RARs, the PPAR subfamily consists of several subtypes. In general, PPARs are lipid sensors activated by fatty acids. However, the PPARβ/δ isotype displays significant affinity for atRA as well; therefore, RA may activate transcription not only through RARs but also through PPARβ/δ (20). In contrast to CRABPII, which transports RA to the nucleus and greatly facilitates RA-RAR ligation and transcriptional activation of RAR-dependent target genes, PPARs interact with fatty acid–binding proteins (FABPs), and in the case of FABP5, the RA-FABP5 complex engages and activates nuclear PPARβ/δ. Therefore, depending on the presence of CRABPII or FABP5, RA can activate either nuclear RARs or PPARβ/δ. Because the binding affinity of CRABPII/RAR for RA is much stronger than for FABP5/PPARβ/δ, RAR-mediated transcriptional activity is dominant. However, higher expression of FABP5 or absence of CRABPII will result in the activation of PPARβ/δ gene transcription in the presence of RA. Given that RA-ligated PPARβ/δ complexes control characteristically antiapoptotic genes, they could overcome the growth-inhibitory activities of RARs and drive survival in the presence of proapoptotic agents (21). Cyclin D3 can also interact with CRABPII in a ligand-independent way, and as a nuclear complex it will enhance the transcriptional activity of RA-ligated RARs (22)."
"The activity of the inflammatory associated transcription factor NF-κB can be repressed by RA []; consequently, NF-κB activation (30) and lipopolysaccharide (LPS)-mediated inflammatory responses (31) are much enhanced under VAD conditions."
"The immune system is greatly influenced by a broad range of effects mediated by retinoids, not only for the development of primary and secondary lymphoid tissues but also for the functional differentiation of various immune cells and the control of inflammatory as well as tolerogenic immune responses. The specific effects RA and its receptors have on the immune response are not always predictable, and depending on the dose, the RAR isoform, the cell type, or the environmental conditions, the outcome can be very different and sometimes paradoxical, suppressing the development of one cell type but promoting at the same time the development of another type or driving immunosuppressive effects in one situation but promoting inflammatory responses in another context. The fact that RA as an exogenous factor directly communicates with nuclear receptors inside the cell, thereby controlling cell fate, provides the immune system with a powerful rheostat to sense the environment and not only control development but also fine-tune the immune response in the most effective and most compatible way to warrant optimal protection with the least damage to the body."
"Retinoids play critical roles during development and differentiation of primary lymphoid organs (44). The thymus, which during embryonic development originates from a pouch of the foregut endodermal tube, may have evolved originally from the primordial gut-associated lymphoid tissue (GALT), which formed the primary lymphoid organ before the appearance of a thymus. As in the intestinal epithelium, RA and RARs are important regulators of thymic epithelial cell homeostasis, and thymic mesenchymal cells are a major source of RA during embryogenesis (45). Furthermore, in developing thymocytes, ligation of RARγ/RXR heterodimers by atRA or 9-cis- RA leads to glucocorticoid-mediated cell death involved in apoptosis by neglect of double-positive (DP) thymocytes. Hence, in vivo treatment with RARγ agonists results in thymic involution due to massive death of DP thymocytes (46, 47). Activation of RARα by large doses of atRA can override RARγ-induced death, whereas strong costimulation of RXR by 9-cis-RA neutralizes the inhibitory effect of RARα (46)."
"Although critical for embryonic development and lymphopoiesis, RA and RARs are first and foremost effective at controlling postnatal immune functions, especially at the mucosal border of the intestine. Flexibility to balance between optimal protective immunity and effective peripheral tolerance is a prerequisite for the mucosal immune system that operates at this largest interface of the body and is thus exposed to numerous pathogen-derived antigens but at the same time is continuously exposed to a vast load of antigens from the diet and commensals that need to be tolerated. Aberrant immune responses directed towards such innocuous antigens can cause local chronic inflammation that often spreads systemically. Consequently, optimal protective immunity has to coincide with effective immune regulation, and RA and its receptors play critical roles in maintaining this balance. Although RA is typically associated with immunosuppressive roles, RA and RARs can also function as initiators of inflammatory responses and protective immunity. Because of this flexibility, these molecules are ideal to control mucosal immune responses and immunity in general; therefore, just about every immune cell is profoundly affected in one way or the other by the signals and molecular mechanisms controlled by RA and its receptors."
"Besides inducing RALDH2 expression, RA influences the maturation and antigen-presentation function of DCs and their ability to prime T cells in either a tolerogenic or an inflammatory fashion."
"Paradoxically, although [] RA-based regulatory functions are all critical to maintain immune homeostasis and warrant steady state immune tolerance, RA also mediates proinflammatory effects on DCs and promotes proinflammatory priming of T cells during an infection or under inflammatory conditions. Furthermore, instead of promoting apoptosis of antigen-presenting DCs, RAR/RXR signaling in conjunction with inflammatory stimuli can lead to survival and maturation of inflammatory DCs, which then upregulate their production of RA in a feedback-loop system and promote increased RA signaling at sites of inflammation (72). RA can further cooperate with inflammatory cytokines such as IL-15 to enhance the production of IL-12p70 and IL-23 by DCs (73) (Figure 3)."
"The effect of RA on macrophages, similar to its effect on DCs, can be anti- or proinflammatory. Upon LPS stimulation, for example, RA decreases the production of IL-12, TNF-α, nitric oxide (NO), PGE2, or COX2 (75–78), whereas it enhances the production of IL-10 (77). Furthermore, IL-10 can feed back on the macrophages and further limit their production of proinflammatory cytokines (79). Alternatively, RA can cooperate with GM-CSF to promote the phagocytic antigen presentation function of macrophages leading to induced regulatory T cell (Treg) formation and immune tolerance (65). In contrast, ligation of RARγ in macrophages controls optimal production of inflammatory cytokines in response to microbial stimuli resulting in a more effective pathogen control, as observed in a Mycobacterium tuberculosis infection (80). Overall, these observations indicate that RA and RAR signaling in mucosal APCs greatly affects the course of the immuneresponse, underscoring the central role of RA in controlling the balance between mucosal immunetolerance and protective immunity."
"The effects of RA signaling in T cells, similar to their effects on the APCs, are context dependent and can be promoting or inhibiting depending on the local and/or intracellular dose of RA, the expression of specific RAR isoforms, the specific cytokine environment, and the nature of the responding T cell."
"Despite its role in the reciprocal regulation of enhanced iTreg generation but suppressed Th17 cell differentiation, RA at low doses is also essential for the functional differentiation of effector T cells. In addition to impaired homing to the site of inflammation, differentiation of effector CD4 T cells is also defective in VAD mice or mice deficient for RARα (43). Furthermore, IFN-γ and IL-17A production by Th1 and Th17 cells in response to an infection was reduced in the absence of RA signaling in T cells (43). Similar results were observed in mouse models of vaccination or allogeneic graft rejection when transcriptional activity of RARs was specifically ablated in CD4 T cells (106). Although linked to the pathogenesis of autoimmune and excessive inflammatory responses, Th17 cells are equally important for protective immunity at the mucosal interface of the intestine, and they are typically present at steady state in this compartment, but they are virtually ablated in VAD mice (43). The ability of APCs to produce IL-6 is also reduced in VAD mice (43), and in a proinflammatory context involving cytokines, such as IL-15, RA acts through DCs to block conversion into Tregs and to promote Th1 effector polarization (73). Therefore, the ability of RA to support effector differentiation probably results from combined actions on DCs and T cells, because addition of RA to Th17 cell–polarizing conditions in APC-less cultures did not enhance effector polarization (107)."
"[..]although mucus plays a critical role in protecting the intestinal barrier, vitamin A has a negative effect on mucus production by goblet cells, which conversely increased under VAD conditions (160). [¿] Enhanced mucus production is further promoted by IL-13 produced by the expanded ILC2 population in the absence of vitamin A (160). Therefore, although an insufficiency of vitamin A greatly impairs the adaptive Th2 response, it equally increases the passive barrier protection and the innate ILC2 response and thus maintains or even increases control of nematode infections (160) (Figure 3). The balanced compensation of RA/RAR signaling in the innate and adaptive immune system allow for flexibility and warrant effective protective immunity adjustable to the circumstances or environmental triggers."
- Retinoic Acid and Immunity (same boss)
"Despite the regulatory properties of RA mentioned [], the action of RA produced by [] different cell types in the gut strongly depends on the physiopathological context []. For example, a study conducted with samples from patients with Crohn’s disease, a very severe inflammatory bowel disease, reported that RAR signalling promotes the differentiation of TNFα-producing inflammatory macrophages and that macrophages from the intestinal mucosa of Crohn’s disease patients are more potent to produce RA (Sanders et al., 2014)."
"Vitamin A and RA promote the differentiation of CD4 helper T cells towards the Th2 phenotype over Th1. Consequently, high-level dietary vitamin A enhanced and VAD diet reduced the development of experimental asthma in a mouse model (Schuster et al., 2008). Although this effect of RA seems to be mainly indirect, via its action on DCs, the use of specific agonists and antagonists revealed that RA, via RARα, directly suppresses Th1 development and enhances Th2 differentiation in murine (Iwata et al., 2003) and human CD4 T cells (Dawson et al., 2008)."
"Although the reciprocal role of RA on Treg and Th17 cell differentiation has been extensively studied, the effect of RA on Th17 differentiation or on already differentiated Th17 seems to depend on the available concentration of RA in the milieu, as well as on the inflammatory/steady-state context. Indeed, several studies have reported that RA could promote Th17 differentiation while in other reports, RA treatment significantly reduced the number of Th17 (Larange and Cheroutre, 2016). The available concentration of RA, the induction of gut homing receptors on Th17 cells and the environmental context could explain these discrepancies (Takahashi et al., 2012; Wang et al., 2010). RA and RARα also play a central role in the phenotype maintenance of already differentiated Th1 cells, by repressing the Th17 differentiation program (Brown et al., 2015) (Figure 4)."
"In addition to its broad ability to confer gut tropism, RA exerts multiple and sometimes paradoxical functions on virtually all types of immune cells, placing this nutrient-derived factor as a central regulator of the immune response. Unlike some unilaterally decisive factors that will systematically steer the immune response in one direction, RA acts more as an adjuvant that will regulate the immune system depending on the physiopathological context, sometimes pushing the balance towards the regulatory side and sometimes towards the inflammatory side. The proper action of RA is guaranteed by the tight regulation of RA metabolism (both organ and cell-type dependent), as well as by the multiplicity of mechanisms of action of RARs, that in addition to their transcriptional functions interfere with many signalling pathways in the nuclear and cytosolic compartments of a cell."
"Vitamin A and RA promote the differentiation of CD4 helper T cells towards the Th2 phenotype over Th1. Consequently, high-level dietary vitamin A enhanced and VAD diet reduced the development of experimental asthma in a mouse model (Schuster et al., 2008). Although this effect of RA seems to be mainly indirect, via its action on DCs, the use of specific agonists and antagonists revealed that RA, via RARα, directly suppresses Th1 development and enhances Th2 differentiation in murine (Iwata et al., 2003) and human CD4 T cells (Dawson et al., 2008)."
"Although the reciprocal role of RA on Treg and Th17 cell differentiation has been extensively studied, the effect of RA on Th17 differentiation or on already differentiated Th17 seems to depend on the available concentration of RA in the milieu, as well as on the inflammatory/steady-state context. Indeed, several studies have reported that RA could promote Th17 differentiation while in other reports, RA treatment significantly reduced the number of Th17 (Larange and Cheroutre, 2016). The available concentration of RA, the induction of gut homing receptors on Th17 cells and the environmental context could explain these discrepancies (Takahashi et al., 2012; Wang et al., 2010). RA and RARα also play a central role in the phenotype maintenance of already differentiated Th1 cells, by repressing the Th17 differentiation program (Brown et al., 2015) (Figure 4)."
"In addition to its broad ability to confer gut tropism, RA exerts multiple and sometimes paradoxical functions on virtually all types of immune cells, placing this nutrient-derived factor as a central regulator of the immune response. Unlike some unilaterally decisive factors that will systematically steer the immune response in one direction, RA acts more as an adjuvant that will regulate the immune system depending on the physiopathological context, sometimes pushing the balance towards the regulatory side and sometimes towards the inflammatory side. The proper action of RA is guaranteed by the tight regulation of RA metabolism (both organ and cell-type dependent), as well as by the multiplicity of mechanisms of action of RARs, that in addition to their transcriptional functions interfere with many signalling pathways in the nuclear and cytosolic compartments of a cell."
A different version of the previous figure.

Blossom, it's possible that your experiencing some of these when you ingest plenty of poison/"vitamin" A.
Blossom, it's possible that your experiencing some of these when you ingest plenty of poison/"vitamin" A.
- Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells (check out figures)
"Retinoic acid (RA), the main biologically active metabolite of vitamin A, has been shown to modulate responses of various immune cells [10]. For example, RA enhances expression of the gut-homing integrin a4ß7 in both murine and human B cells and CD4+ T cells [11–13]. Moreover, RA cooperates with TGF-ß to promote the conversion of naive CD4+ T cells into Foxp3+ Treg cells in mice as well as humans [14, 15]. In contrast, RA has been shown to activate effector CD4+ T cells under proinflammatory conditions. The RA-retinoic acid receptor a (RARa) axis is essential for the production of the proinflammatory cytokines IFN-y and IL-17A by Th1 and Th17 cells in response to infection [16]. Furthermore, RA enhances Th2 responses in human CD4+ T cells in vitro and in helminth-infected mice [12, 17–19]. A few recent studies have reported an effect of RA on murine ILCs. Mielke et al. showed that RA promotes production of IL-22 in ILC3 cells stimulated with IL-1ß and IL-23 [20]. In addition, Van de Pavert et al. observed that fetal RA signaling controls the differentiation of LTi cells, which are a subset of ILC3 cells and crucial for the formation of secondary lymphoid organs. The authors established that maternal levels of dietary vitamin A control the size of secondary lymphoid organs and the efficiency of immune responses in the adult offspring [21]."
"In the present study, we hypothesized that RA and 1,25D3 influence human ILC responses. Because our primary interest is in allergy, we put emphasis on ILC2 cells. We observed that RA acts synergistically with IL-2 and other cytokines known to stimulate ILCs, to induce expression of the gut-homing integrin a4ß7, as well as production of IL-5 and IL-13 in ILC2 cells, and IFN-y in ILC1 and ILC3 cells. In contrast, 1,25D3 inhibited the effects of RA on cytokine production and expression of gut-homing integrin. Together, these data shed a light on the effect of two immunomodulatory vitamins on human ILC responses. The balance between these vitamins, which has shifted with industrialization, may be an important factor in the development of allergic inflammation and other chronic immune disorders in which ILCs play a role."
"Allergic and other chronic immune-mediated diseases have become rapidly more prevalent in association with modernization and urbanization around the world [29–31]. There are myriad environmental changes suggested to partially explain this association. The ‘vitamin hypothesis’ posits that nutritional shifts accompanying these lifestyle changes, including increasing vitamin A sufficiency and vitamin D deficiency, may contribute to this trend by influencing conventional CD4+ T cell, B cell and basophil responses [22, 24, 32]. Our findings presented here suggest that ILCs may also be influenced in a pro-inflammatory manner by those changes."
"AD [Atopic Dermatitis] is often the first manifestation of the ‘atopic march’, and approximately one-third of young children with moderate-to-severe AD develop food allergies, which are frequently participating triggers of the inflammation [37]. An important hallmark of AD is a defective epidermal barrier function, which allows the penetration of environmental triggers such as allergens, and their interaction with the immune system."
"We observed that 1,25D3 inhibits RA-induced production of effector cytokines and expression of gut-homing integrin in human ILCs. ILC2 cells have been shown to enhance allergic inflammation, and two recent studies reported that ILC2 cells also facilitate sensitization to allergens [6, 35, 42, 45–47]. Inhibition of ILC2 cell activation and gut homing by 1,25D3 could therefore ameliorate allergic inflammation, particularly in the gastro-intestinal tract, and perhaps in this way play a role in prevention of allergic disease."
"In summary, we have shown that the biologically active metabolites of vitamins A and D have profound effects on cytokine production and homing of human ILCs. Our data provide additional evidence for a mechanism by which alterations in nutrition and exposure to sunlight may influence immunity."
"In the present study, we hypothesized that RA and 1,25D3 influence human ILC responses. Because our primary interest is in allergy, we put emphasis on ILC2 cells. We observed that RA acts synergistically with IL-2 and other cytokines known to stimulate ILCs, to induce expression of the gut-homing integrin a4ß7, as well as production of IL-5 and IL-13 in ILC2 cells, and IFN-y in ILC1 and ILC3 cells. In contrast, 1,25D3 inhibited the effects of RA on cytokine production and expression of gut-homing integrin. Together, these data shed a light on the effect of two immunomodulatory vitamins on human ILC responses. The balance between these vitamins, which has shifted with industrialization, may be an important factor in the development of allergic inflammation and other chronic immune disorders in which ILCs play a role."
"Allergic and other chronic immune-mediated diseases have become rapidly more prevalent in association with modernization and urbanization around the world [29–31]. There are myriad environmental changes suggested to partially explain this association. The ‘vitamin hypothesis’ posits that nutritional shifts accompanying these lifestyle changes, including increasing vitamin A sufficiency and vitamin D deficiency, may contribute to this trend by influencing conventional CD4+ T cell, B cell and basophil responses [22, 24, 32]. Our findings presented here suggest that ILCs may also be influenced in a pro-inflammatory manner by those changes."
"AD [Atopic Dermatitis] is often the first manifestation of the ‘atopic march’, and approximately one-third of young children with moderate-to-severe AD develop food allergies, which are frequently participating triggers of the inflammation [37]. An important hallmark of AD is a defective epidermal barrier function, which allows the penetration of environmental triggers such as allergens, and their interaction with the immune system."
"We observed that 1,25D3 inhibits RA-induced production of effector cytokines and expression of gut-homing integrin in human ILCs. ILC2 cells have been shown to enhance allergic inflammation, and two recent studies reported that ILC2 cells also facilitate sensitization to allergens [6, 35, 42, 45–47]. Inhibition of ILC2 cell activation and gut homing by 1,25D3 could therefore ameliorate allergic inflammation, particularly in the gastro-intestinal tract, and perhaps in this way play a role in prevention of allergic disease."
"In summary, we have shown that the biologically active metabolites of vitamins A and D have profound effects on cytokine production and homing of human ILCs. Our data provide additional evidence for a mechanism by which alterations in nutrition and exposure to sunlight may influence immunity."
- Retinoic Acid and Its Role in Modulating Intestinal Innate Immunity
"Given the recent findings on the role of VA affecting both the innate and adaptive arms of the immune system, it seems plausible to implicate VA deficiency in increased susceptibility towards infectious diseases [7,8]. On the other hand, VA excess leads to deregulation of liver metabolic functions and presents several other toxic effects [9]. The role of VA metabolite atRA in adaptive immune responses has been extensively reviewed elsewhere [10,11,12], particularly in the context of intestinal immunity. Here, we review the recent findings where atRA plays a central role in the development and functioning of the innate arm of the immune system, in particular the myeloid compartment and innate lymphoid cells (ILCs)."
"Besides being the primary site for VA absorption, intestinal epithelial cells (IECs) can metabolize VA to RA by their ability to express ALDH1A1 [19,20]."
"After differentiation and migration to the intestine, specific DC subsets are endowed with the ability to sense and respond to atRA [45]. The general consensus on the effect of atRA on DC function is to promote an anti-inflammatory phenotype characteristic of intestinal DCs [46,47]. Transcriptional profiling of in vitro differentiated DCs in the presence of atRA showed downregulation of pro-inflammatory genes involved in the NF-kB mediated inflammatory program [45]. However, besides the above-mentioned immuno-regulatory effects, some studies have emphasized a rather unorthodox pro-inflammatory role of atRA in DCs. In the presence of IL-15, atRA was shown to act as an adjuvant in promoting the secretion of the pro-inflammatory cytokines IL-12 and IL-23 by DCs [48]. Similarly, human DCs generated from monocytes in the presence of atRA primarily induced interferon gamma (IFNy) production by CD4+ T cells in vitro [49]. The dual role of atRA in influencing DCs function might be a result of the microenvironments and/or cytokine milieu to which the DCs are exposed. For example, atRA in a cytokine environment that is either pro- or anti-inflammatory would induce a tolerogenic or pro-inflammatory DC phenotype, respectively."
"Besides being able to sense and get influenced by atRA, DCs, in turn, are one of the main producers of atRA, a phenomenon that has been well-studied in intestinal CD103+ DCs expressing high levels of the Aldh1a2 gene. This is most evident in the intestine where CD103+ DCs in the proximal tract of the small intestine are exposed to higher levels of atRA, which, in turn, renders them better atRA producers compared to distal small intestinal or colonic DCs. However, this is not limited to the intestine since extra intestinal DCs endowed with the ability to produce atRA have also been described, such as CD103- DCs in the skin [41]. Importantly, CD103- DCs in the skin can induce Foxp3+ TREG similar to intestinal CD103+ DCs [41]. Several factors can influence DCs into atRA-producing cells. For example, incubation of DCs with atRA alone or in combination with various cytokines such as interleukin-4 (IL-4) and transforming growth factor beta (TGFß) cytokines were able to induce Aldh1a2 mRNA expression, which seems to be sufficient to render DCs with the capacity to metabolize atRA [50,51,52]. In addition, the short chain fatty acid butyrate was shown to induce Aldh1a expression in monocyte-derived dendritic cells, suggesting that microbiota-derived metabolites might play important roles in intestinal DC function [53]. Moreover, the Wnt pathway seems to be crucial in imprinting intestinal DCs since this pathway seems to be crucial to induce Aldh1a2 expression and produce atRA [54]. Furthermore, MyD88 signaling and, in particular, toll-like receptor (TLR) 1/2 stimulation can also induce Aldh1a2 mRNA expression and imprint extra-intestinal DCs with atRA producing capacity [15,55,56]. While multiple factors and pathways have been shown to influence the atRA producing capacity of DCs, whether two or more of these pathways interact with each other needs to be investigated. Irrespective of the mechanism, DCs imprinted to produce atRA primarily induce a tolerogenic program by generation of Foxp3 TREG cells. Importantly, atRA production by intestinal CD103+ DCs seems to be crucial for the generation of TREG and for the establishment of tolerance towards innocuous dietary antigens, a process known as oral tolerance."
"Local and systemic immune unresponsiveness towards antigens that have been previously administered by oral route is classically defined as oral tolerance [12,57]. These antigens, which are considered innocuous, are primarily derived from diet and failure to mount an effective tolerance towards these antigens has been associated with conditions such as food allergies and celiac disease. VA has proven crucial to the establishment of oral immunological tolerance against food antigens [12,57], and its deficiency might contribute towards food allergies, celiac disease [¿] and inflammatory bowel diseases (IBD). Indeed, mice deprived of VA resulted in exacerbated intestinal inflammation as assessed by colitis score and colon length using the dextran sodium sulfate (DSS)-induced colitis model [58]. Moreover, atRA supplementation efficiently attenuates chronic inflammation in a mouse model of ileitis [59]. Notably, atRA participates in more than one of the five-step models proposed for the establishment of oral immunological tolerance [12] (Figure 2)."
"It is well established that the concentrations of atRA in the small intestine follows a proximal (i.e., duodenum) to distal (i.e., colon) decreasing gradient [15,23]. Similarly, ILC subsets are differentially distributed along the gastrointestinal tract with ILC3 outnumbering ILC1 and ILC2 in the small intestine, whereas ILC2 are predominant in the colon [78]. Therefore, it is tempting to speculate that atRA might be one of the players involved in the complex functional regionalization of ILCs in the intestine, likely by controlling their migration, differentiation and/or function."
"Recent studies support the role of atRA as an important enhancer of DC differentiation and migration from the bone marrow to the intestine, ultimately resulting in DCs that are capable of both sensing and producing atRA. In addition, atRA works as a crucial regulator of DC function, which dictates T helper and effector cell function in the mucosal sites and in peripheral tissues. Interestingly, the cytokine milieu can influence DCs to induce pro-inflammatory T helper functions, even in the presence of atRA. Finally, recent advances in the ILC field point towards atRA-producing DCs as modulators of ILC migration, function and phenotype plasticity. Taken together, atRA exerts a crucial role in DC function in order to maintain tolerance against food and microbial antigens and promote tissue homeostasis."
"Besides being the primary site for VA absorption, intestinal epithelial cells (IECs) can metabolize VA to RA by their ability to express ALDH1A1 [19,20]."
"After differentiation and migration to the intestine, specific DC subsets are endowed with the ability to sense and respond to atRA [45]. The general consensus on the effect of atRA on DC function is to promote an anti-inflammatory phenotype characteristic of intestinal DCs [46,47]. Transcriptional profiling of in vitro differentiated DCs in the presence of atRA showed downregulation of pro-inflammatory genes involved in the NF-kB mediated inflammatory program [45]. However, besides the above-mentioned immuno-regulatory effects, some studies have emphasized a rather unorthodox pro-inflammatory role of atRA in DCs. In the presence of IL-15, atRA was shown to act as an adjuvant in promoting the secretion of the pro-inflammatory cytokines IL-12 and IL-23 by DCs [48]. Similarly, human DCs generated from monocytes in the presence of atRA primarily induced interferon gamma (IFNy) production by CD4+ T cells in vitro [49]. The dual role of atRA in influencing DCs function might be a result of the microenvironments and/or cytokine milieu to which the DCs are exposed. For example, atRA in a cytokine environment that is either pro- or anti-inflammatory would induce a tolerogenic or pro-inflammatory DC phenotype, respectively."
"Besides being able to sense and get influenced by atRA, DCs, in turn, are one of the main producers of atRA, a phenomenon that has been well-studied in intestinal CD103+ DCs expressing high levels of the Aldh1a2 gene. This is most evident in the intestine where CD103+ DCs in the proximal tract of the small intestine are exposed to higher levels of atRA, which, in turn, renders them better atRA producers compared to distal small intestinal or colonic DCs. However, this is not limited to the intestine since extra intestinal DCs endowed with the ability to produce atRA have also been described, such as CD103- DCs in the skin [41]. Importantly, CD103- DCs in the skin can induce Foxp3+ TREG similar to intestinal CD103+ DCs [41]. Several factors can influence DCs into atRA-producing cells. For example, incubation of DCs with atRA alone or in combination with various cytokines such as interleukin-4 (IL-4) and transforming growth factor beta (TGFß) cytokines were able to induce Aldh1a2 mRNA expression, which seems to be sufficient to render DCs with the capacity to metabolize atRA [50,51,52]. In addition, the short chain fatty acid butyrate was shown to induce Aldh1a expression in monocyte-derived dendritic cells, suggesting that microbiota-derived metabolites might play important roles in intestinal DC function [53]. Moreover, the Wnt pathway seems to be crucial in imprinting intestinal DCs since this pathway seems to be crucial to induce Aldh1a2 expression and produce atRA [54]. Furthermore, MyD88 signaling and, in particular, toll-like receptor (TLR) 1/2 stimulation can also induce Aldh1a2 mRNA expression and imprint extra-intestinal DCs with atRA producing capacity [15,55,56]. While multiple factors and pathways have been shown to influence the atRA producing capacity of DCs, whether two or more of these pathways interact with each other needs to be investigated. Irrespective of the mechanism, DCs imprinted to produce atRA primarily induce a tolerogenic program by generation of Foxp3 TREG cells. Importantly, atRA production by intestinal CD103+ DCs seems to be crucial for the generation of TREG and for the establishment of tolerance towards innocuous dietary antigens, a process known as oral tolerance."
"Local and systemic immune unresponsiveness towards antigens that have been previously administered by oral route is classically defined as oral tolerance [12,57]. These antigens, which are considered innocuous, are primarily derived from diet and failure to mount an effective tolerance towards these antigens has been associated with conditions such as food allergies and celiac disease. VA has proven crucial to the establishment of oral immunological tolerance against food antigens [12,57], and its deficiency might contribute towards food allergies, celiac disease [¿] and inflammatory bowel diseases (IBD). Indeed, mice deprived of VA resulted in exacerbated intestinal inflammation as assessed by colitis score and colon length using the dextran sodium sulfate (DSS)-induced colitis model [58]. Moreover, atRA supplementation efficiently attenuates chronic inflammation in a mouse model of ileitis [59]. Notably, atRA participates in more than one of the five-step models proposed for the establishment of oral immunological tolerance [12] (Figure 2)."
"It is well established that the concentrations of atRA in the small intestine follows a proximal (i.e., duodenum) to distal (i.e., colon) decreasing gradient [15,23]. Similarly, ILC subsets are differentially distributed along the gastrointestinal tract with ILC3 outnumbering ILC1 and ILC2 in the small intestine, whereas ILC2 are predominant in the colon [78]. Therefore, it is tempting to speculate that atRA might be one of the players involved in the complex functional regionalization of ILCs in the intestine, likely by controlling their migration, differentiation and/or function."
"Recent studies support the role of atRA as an important enhancer of DC differentiation and migration from the bone marrow to the intestine, ultimately resulting in DCs that are capable of both sensing and producing atRA. In addition, atRA works as a crucial regulator of DC function, which dictates T helper and effector cell function in the mucosal sites and in peripheral tissues. Interestingly, the cytokine milieu can influence DCs to induce pro-inflammatory T helper functions, even in the presence of atRA. Finally, recent advances in the ILC field point towards atRA-producing DCs as modulators of ILC migration, function and phenotype plasticity. Taken together, atRA exerts a crucial role in DC function in order to maintain tolerance against food and microbial antigens and promote tissue homeostasis."
- Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases
"[..]after the absorption and metabolization of vitamin A into RA in the gut, RA plays critical roles in the mucosal immune response as a regulatory signal in the intestinal mucosa by promoting Foxp3 regulatory T cell differentiation [6] and immunoglobulin (Ig) A production [7]. In addition, RA induces the homing of innate immune cells, such as innate lymphoid cells (ILCs) [8] besides regulatory and effector T and B cells, to the gut [9–11]. During infections, RA can induce the production of proinflammatory cytokines by dendritic cells (DCs), promoting the differentiation of effector T cells and the protection of the mucosa [12]. Thus, RA is crucial for maintaining homeostasis at the intestinal barrier and equilibrating immunity and tolerance. Due to the extensive role of RA in immune cells and the immune response, reducing mortality in children by vitamin A supplementation may be possible [4]."
"In addition, due to its regulatory activity, RA has been shown to play an important role in the control of inflammatory diseases not only in the intestine [13, 14] but also in other tissues, such as the central nervous system [15–17] and pulmonary mucosa [18, 19]."
"After uptake, RA is generated from retinol by two sequential reactions. In the first reversible reaction, retinol is oxidized into retinal by the ubiquitously expressed enzyme alcohol dehydrogenase (ADH) [20]. Subsequently, in intestinal epithelium cells, DCs and macrophages associated with mesenteric lymph nodes (mLNs) and Peyer’s patches (PPs), retinal is oxidized by the enzyme retinal dehydrogenase (RALDH) to generate RA [1]. There are three isoforms of RALDH (RALDH1, RALDH2, and RALDH3) [21], and [again,] their expression is tightly regulated and limited in the cells mentioned above. Thus, RALDH is considered the main enzyme that defines the populations of cells that are capable of producing RA [20]. Intestinal epithelium cells can also metabolize vitamin A after absorption into retinal and RA, which can be directly released into the intestinal mucosa [21]."
"RA can be generated in multiple forms as all-trans, 9-cis, and 13-cis RA [26, 27]; however, all-trans RA (atRA) is physiologically the most abundant [28]. RA interacts with nuclear receptors, such as the retinoic acid receptor (RAR) and retinoid receptor X (RXR), to regulate the transcription of several target genes [10, 29] by binding the retinoic acid-responsive elements (RAREs) in DNA [30]. These receptors form heterodimers; RAR comprises three major isoforms (a, b, and y) that interact with all forms of RA, whereas RXR, which also has the a, b, and y isoforms, mainly interacts with 9-cis RA [31]. RA can also signal through peroxisome proliferator-activating receptor beta (PPAR-ß) when it forms a heterodimer with RXR, which may be important for lipid metabolism and glucose homeostasis [1]."
"Control of the RA concentration in tissues is performed by a group of enzymes that belong to the cytochrome P450 family 26 (CYP26), including subfamilies A1, B1, and C1 (CYP26A1, CYP26B1, and CYP26C1), which catalyze RA present in the cytosol to generate the oxidized forms (5,8-epoxy RA, 4-oxo RA, 4-hydroxy RA, and 18-hydroxy RA) [34, 35]. The action of these enzymes prevents RA accumulation in the organism and maintains optimal physiological RA concentrations for the best performance."
"Some inflammatory factors may influence RALDH expression, such as prostaglandin E2 (PGE2), which is produced by peripheral stromal cells and suppresses the differentiation of RA+ DCs by directly antagonizing RALDH expression [50]. In addition, DCs that infiltrate the gut during inflammation do not acquire RALDH activity, which is required for RA synthesis. These inflammatory DCs express E-cadherin and the CD103 receptor, accumulate in the mesenteric lymph nodes and the inflamed colon, exhibit high expression of Toll-like receptors (TLRs) and produce cytokines IL-6 and IL-23, enhancing inflammation [47]."
"In infections, RA signaling may also induce the production of proinflammatory cytokines by DCs, promoting the differentiation of effector T cells [12] and enhancing the cellular activation state, in addition to the promotion of the formation of tertiary lymphoid structures [10]. These structures are formed in response to nonresolving inflammation generating lymphoid aggregates that drive adaptive immune reactions [54, 55]. RA also influences the maturation of monocyte-derived DCs (MoDCs) by increasing the expression of major histocompatibility complex (MHC) class II and CD86 and regulating the survival of DCs via the RARa/RXR pathway [56]. In parallel to the activation of the innate immune response, RA promotes human DCs to induce IL-10-producing T cells to control inflammation and the maintenance of tissue homeostasis [57]."
"In addition, due to its regulatory activity, RA has been shown to play an important role in the control of inflammatory diseases not only in the intestine [13, 14] but also in other tissues, such as the central nervous system [15–17] and pulmonary mucosa [18, 19]."
"After uptake, RA is generated from retinol by two sequential reactions. In the first reversible reaction, retinol is oxidized into retinal by the ubiquitously expressed enzyme alcohol dehydrogenase (ADH) [20]. Subsequently, in intestinal epithelium cells, DCs and macrophages associated with mesenteric lymph nodes (mLNs) and Peyer’s patches (PPs), retinal is oxidized by the enzyme retinal dehydrogenase (RALDH) to generate RA [1]. There are three isoforms of RALDH (RALDH1, RALDH2, and RALDH3) [21], and [again,] their expression is tightly regulated and limited in the cells mentioned above. Thus, RALDH is considered the main enzyme that defines the populations of cells that are capable of producing RA [20]. Intestinal epithelium cells can also metabolize vitamin A after absorption into retinal and RA, which can be directly released into the intestinal mucosa [21]."
"RA can be generated in multiple forms as all-trans, 9-cis, and 13-cis RA [26, 27]; however, all-trans RA (atRA) is physiologically the most abundant [28]. RA interacts with nuclear receptors, such as the retinoic acid receptor (RAR) and retinoid receptor X (RXR), to regulate the transcription of several target genes [10, 29] by binding the retinoic acid-responsive elements (RAREs) in DNA [30]. These receptors form heterodimers; RAR comprises three major isoforms (a, b, and y) that interact with all forms of RA, whereas RXR, which also has the a, b, and y isoforms, mainly interacts with 9-cis RA [31]. RA can also signal through peroxisome proliferator-activating receptor beta (PPAR-ß) when it forms a heterodimer with RXR, which may be important for lipid metabolism and glucose homeostasis [1]."
"Control of the RA concentration in tissues is performed by a group of enzymes that belong to the cytochrome P450 family 26 (CYP26), including subfamilies A1, B1, and C1 (CYP26A1, CYP26B1, and CYP26C1), which catalyze RA present in the cytosol to generate the oxidized forms (5,8-epoxy RA, 4-oxo RA, 4-hydroxy RA, and 18-hydroxy RA) [34, 35]. The action of these enzymes prevents RA accumulation in the organism and maintains optimal physiological RA concentrations for the best performance."
"Some inflammatory factors may influence RALDH expression, such as prostaglandin E2 (PGE2), which is produced by peripheral stromal cells and suppresses the differentiation of RA+ DCs by directly antagonizing RALDH expression [50]. In addition, DCs that infiltrate the gut during inflammation do not acquire RALDH activity, which is required for RA synthesis. These inflammatory DCs express E-cadherin and the CD103 receptor, accumulate in the mesenteric lymph nodes and the inflamed colon, exhibit high expression of Toll-like receptors (TLRs) and produce cytokines IL-6 and IL-23, enhancing inflammation [47]."
"In infections, RA signaling may also induce the production of proinflammatory cytokines by DCs, promoting the differentiation of effector T cells [12] and enhancing the cellular activation state, in addition to the promotion of the formation of tertiary lymphoid structures [10]. These structures are formed in response to nonresolving inflammation generating lymphoid aggregates that drive adaptive immune reactions [54, 55]. RA also influences the maturation of monocyte-derived DCs (MoDCs) by increasing the expression of major histocompatibility complex (MHC) class II and CD86 and regulating the survival of DCs via the RARa/RXR pathway [56]. In parallel to the activation of the innate immune response, RA promotes human DCs to induce IL-10-producing T cells to control inflammation and the maintenance of tissue homeostasis [57]."
- Retinoic Acid, Leaky Gut, and Autoimmune Diseases (@Tarmander - 3.1/4.3.1)
- Retinoic acid syndrome: a review (massive amounts given in cancre, perhaps there's something to learn from it)
- Vitamin A-Deficient Hosts Become Nonsymptomatic Reservoirs of Escherichia coli-Like Enteric Infections
--
Complementing post #1693..
- Inflamed About Retinoic Acid
↳ Context is key in the gut (don't be fooled by the title)
- Reciprocal TH17 and Regulatory T Cell Differentiation Mediated by Retinoic Acid
- Paradoxical Effects of All-Trans-Retinoic Acid on Lupus-Like Disease in the MRL/lpr Mouse Model
--
Propoison A:
- Concentrations of Selected Carotenoids and Vitamin A in Human Liver, Kidney and Lung Tissue
- Mammalian carotenoid absorption and metabolism
"Carotenoids are ubiquitous in mammalian tissues and the accumulation of carotenoids among animals is species specific and highly variable [64]. These differences could be explained by dietary intake and/or species differences in absorption and metabolism, such as gut motility or handling of lipoproteins by the body. Goodwin [65] classified groups of animals into carotenoid accumulators, non-accumulators, and intermediates. Primates, similarly to humans, accumulate a wide range of carotenoids in high concentrations, while other mammalian species accumulate only one specific carotenoid/class of carotenoid or no carotenoids at all. Birds preferentially accumulate xanthophylls in most tissues [64]. Contrary to past studies, Chew et al. [66] recently reported that the domestic cat absorbs significant amounts of b-carotene from dietary sources. Rats, gerbils, ferrets, and preruminant calves absorb and/or accumulate carotenoids in tissues to varying degrees and thus are animal models used to study absorption and metabolism [67,68]."
[68] Review of Animal Models in Carotenoid Research
[68] Review of Animal Models in Carotenoid Research
- Carotenoids: Linking Chemistry, Absorption, and Metabolism to Potential Roles in Human Health and Disease
"Once in the blood, carotenoids are passively taken up into various tissues following degradation of lipoproteins by lipoprotein lipase (LPL; see Fig. 5). Chylomicron remnants are cleared from the blood in the liver by the chylomicron receptor. Carotenoids accumulate in the liver, but regulation of their storage is poorly understood. Recently, Rao and co-workers (4) purified a cellular carotenoid-binding protein (CCBP) from ferret liver, which had a high degree of specificity toward carotenoids with at least one β-ionone ring, but not toward other carotenoids. This group proposed that CCBP may play a role in storage, transport, and metabolism of provitamin A carotenoids, as well as act as a natural substrate for metabolic reactions involving these carotenoids (4). Carotenoids may exit the liver into the blood following incorporation into very low-density lipoproteins (VLDL). Subsequent uptake of carotenoids into tissues from VLDL, and especially LDL, is thought to occur through the LDL receptor because the tissues with the highest levels of carotenoids (e.g., liver, adrenal glands, testes) tend to have high LDL receptor activity (128,137,138)."
"Carotenoids are present in many tissues in humans, including liver, adipose, pancreas, kidney, lung, adrenal, spleen, heart, thyroid, testes, ovary (87,139), and eye (140–142). Total quantitative levels of carotenoids are highest in liver and adipose tissue, the main storage sites for carotenoids (128). Concentrations of carotenoids (i.e., per gram of tissue basis) are highest in the liver, adrenal, and reproductive tissues. As is true for serum, β-carotene, lycopene, lutein, α-carotene, zeaxanthin, and cryptoxanthin are the main tissue carotenoids (137,139). In addition, the geometrical isomers of lycopene and β-carotene found in serum are also found in tissues (87). Some tissues exhibit specific patterns of carotenoid accumulation, suggesting that certain carotenoids may exert a biological effect in one tissue over another."
"Carotenoids are present in many tissues in humans, including liver, adipose, pancreas, kidney, lung, adrenal, spleen, heart, thyroid, testes, ovary (87,139), and eye (140–142). Total quantitative levels of carotenoids are highest in liver and adipose tissue, the main storage sites for carotenoids (128). Concentrations of carotenoids (i.e., per gram of tissue basis) are highest in the liver, adrenal, and reproductive tissues. As is true for serum, β-carotene, lycopene, lutein, α-carotene, zeaxanthin, and cryptoxanthin are the main tissue carotenoids (137,139). In addition, the geometrical isomers of lycopene and β-carotene found in serum are also found in tissues (87). Some tissues exhibit specific patterns of carotenoid accumulation, suggesting that certain carotenoids may exert a biological effect in one tissue over another."
- Evidence for compartmentalization of mammalian carotenoid metabolism
- β-Carotene conversion products and their effects on adipose tissue
- A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries)
- Presence of fed β-carotene in digesta, excreta, blood, andhepatic and adipose tissues of Holstein steers
- Carotenoid trade-off between parasitic resistance and sexual display: an experimental study in the blackbird (Turdus merula) (it's cool)
That's understandable.Okay, thanks for encouraging me to think more on this issue.
Upon reflection I can say with certainty that I didn’t consume milk regularly from the age of about 14-43. Occasionally I would have a small amount with cereal but probably less than 5 times per year. I never avoided dairy completely but it wasn’t part of my daily diet and I was still unwell. I developed chronic fatigue syndrome while not consuming dairy. I had used a micellized A around the time I was hit with Epstein Bar virus and resulting CFS. Maybe that’s a coincidence or maybe not. I used retin-a daily from ages 20-40.
The celiac disease complicates things in my case because I was certainly eating gluten multiple times daily for my whole life until age 42. I somewhat improved in 2011 from going gluten free but when I started the Wahl’s Autoimmune Diet which is a paleo approach that calls for abundant colorful fruits and vegetables I became very ill despite no dairy or gluten.
I don’t think casein is my main issue but I could have developed a sensitivity to it from chronic gut inflammation.
When I first discovered Peat I seemed ok with dairy for awhile but I had been gluten free for a couple years at that point so my inflammation was down.
All of that leads me to believe eliminating dairy isn’t the primary reason for my current improvements but it could be a contributing factor.
I’ve consumed it before and tolerated it so I think it’s possible I might be able to again at some point if I decide to test it out.
Right now I’m not motivated to experiment much though because I’ve found a way of eating that doesn’t feel restricted (believe it or not!) and I feel good everyday.
I'm counting on you US and A citizens to start a chain reaction by requesting from your local producers 'The Return of White Carrots'. They should be featured on your Instagram pages as part of artificial dishes, which in turn will influence artificial people from unimportant countries to copy what you're doing, which in turn will lead to improvised pictures using radishes but at the same time create multiple local demands for producers to grow more of the real deal, and we'll conclude the reactions with a comment with hints of hypotyphoid selfishness that it will eventually benefit me for making it easier to buy them. Thank you as well in advance.
Last edited:
White carrots for Uganda!I'm counting on you US and A citizens to start a chain reaction by requesting from your local producers 'The Return of White Carrots'. They should be featured on your Instagram pages as part of artificial dishes, which in turn will influence artificial people from unimportant countries to copy what you're doing, which in turn will lead to improvised pictures using radishes but at the same time create multiple local demands for producers to grow more of the real deal, and we'll conclude the reactions with a comment with hints of hypotyphoid selfishness that it will eventually benefit me for making it easier to buy them. Thank you as well in advance.
InChristAlone
Member
Migraine following a large vitamin A intake is not good!@Blossom You said OJ has vit A? hmm this is interesting.. I don't like OJ that much.. The taste of liver in general disgusts me. Eggs give me bloating problems. Could I be doing low vitamin A diet unintentionally? Two weeks ago, I asked myself "when was I the last time I had liver?" and the answer to my surprise was more than 2 months ago. So I took myself to the restaurant and ordered two grilled lamb liver sandwiches and I had the worst migraine the following day. I was also anti-social when I went for my family lunch gathering. This theory could have some truth even though I don't want to jump to conclusions and I strongly advise people to test the theory by themselves. What do you think of vitamin D supplementation? @Janelle525 do you take vitamin D?
No I have not taken vitamin D in years. I load up during the summer. Apparently vitamin D is a pro hormone and can rise even in winter when getting no source of it, so it must be something the body uses for a purpose and doesn't behave like a vitamin we have to take in everyday.
Tarmander
Member
- Joined
- Apr 30, 2015
- Messages
- 3,772
re the leaky gut study you showed me. If you were following Dr. Smith's rules, when a study shows something is improved by A in the short term, that thing gets totally trashed in the long term. So if RA can help leaky gut in the short term, in the long term....well I would say my experience of allergies and leaky gut matches this pretty well.- Glossary of Immumology terms | Immunopaedia
- Retinoic Acid and Retinoic Acid Receptors as Pleiotropic Modulators of the Immune System
"Although the all-trans RA (atRA) isoform predominates in most tissues, additional isoforms of RA can be detected, such as 13-cis-RA. The specific effects of RA are critically dose dependent and therefore depend on the spatial and temporal regulation of RA metabolism, transportation, and diffusion, which determine the intracellular and tissue-specific concentration gradients."- Retinoic Acid and Immunity (same boss)
"A variant of atRA, 9-cis-RA, is equally capable in activating RARs, but atRA is 50-fold weaker in activating RXRs (15). The biological significance of 9-cis-RA as an RXR ligand remains controversial because 9-cis-RA is hardly detectable in vivo (16). Furthermore, oleic acid and docosahexaenoic acid also bind and activate RXR (9), and given that RXRs also associate with other nuclear receptors that participate in fatty acid biosynthesis, metabolism, or transport, it is conceivable that the true natural ligands of RXR are fatty acids (17). Nevertheless, the activation of RXR as part of the RAR/RXR complex is silenced in the absence of RAR ligands, a phenomenon referred to as RXR subordination. The participation of RXR is mainly to enhance the DNA binding of the heterodimer, and simultaneous ligation of the receptors augments the transcriptional activation of RARs (18)."
"In general, retinoid receptors reside in the nucleus; however, in some cells or under certain conditions, they can be found in the cytoplasm, where they display nongenomic functions in a ligand-dependent or -independent fashion and as monomers or complexed with various cellular factors (14) []. The ability of these receptors to participate in extranuclear signaling cascades adds to the complexity and pleiotropic effects of their ligand-dependent and -independent roles."
"In addition to RARs, RXRs can form heterodimeric complexes with other nuclear receptors, such as the thyroid hormone receptor, the vitamin D3 receptor, the COUP-TFII, and the peroxisome proliferator–activated receptors (PPARs). Like RARs, the PPAR subfamily consists of several subtypes. In general, PPARs are lipid sensors activated by fatty acids. However, the PPARβ/δ isotype displays significant affinity for atRA as well; therefore, RA may activate transcription not only through RARs but also through PPARβ/δ (20). In contrast to CRABPII, which transports RA to the nucleus and greatly facilitates RA-RAR ligation and transcriptional activation of RAR-dependent target genes, PPARs interact with fatty acid–binding proteins (FABPs), and in the case of FABP5, the RA-FABP5 complex engages and activates nuclear PPARβ/δ. Therefore, depending on the presence of CRABPII or FABP5, RA can activate either nuclear RARs or PPARβ/δ. Because the binding affinity of CRABPII/RAR for RA is much stronger than for FABP5/PPARβ/δ, RAR-mediated transcriptional activity is dominant. However, higher expression of FABP5 or absence of CRABPII will result in the activation of PPARβ/δ gene transcription in the presence of RA. Given that RA-ligated PPARβ/δ complexes control characteristically antiapoptotic genes, they could overcome the growth-inhibitory activities of RARs and drive survival in the presence of proapoptotic agents (21). Cyclin D3 can also interact with CRABPII in a ligand-independent way, and as a nuclear complex it will enhance the transcriptional activity of RA-ligated RARs (22)."
"The activity of the inflammatory associated transcription factor NF-κB can be repressed by RA []; consequently, NF-κB activation (30) and lipopolysaccharide (LPS)-mediated inflammatory responses (31) are much enhanced under VAD conditions."
"The immune system is greatly influenced by a broad range of effects mediated by retinoids, not only for the development of primary and secondary lymphoid tissues but also for the functional differentiation of various immune cells and the control of inflammatory as well as tolerogenic immune responses. The specific effects RA and its receptors have on the immune response are not always predictable, and depending on the dose, the RAR isoform, the cell type, or the environmental conditions, the outcome can be very different and sometimes paradoxical, suppressing the development of one cell type but promoting at the same time the development of another type or driving immunosuppressive effects in one situation but promoting inflammatory responses in another context. The fact that RA as an exogenous factor directly communicates with nuclear receptors inside the cell, thereby controlling cell fate, provides the immune system with a powerful rheostat to sense the environment and not only control development but also fine-tune the immune response in the most effective and most compatible way to warrant optimal protection with the least damage to the body."
"Retinoids play critical roles during development and differentiation of primary lymphoid organs (44). The thymus, which during embryonic development originates from a pouch of the foregut endodermal tube, may have evolved originally from the primordial gut-associated lymphoid tissue (GALT), which formed the primary lymphoid organ before the appearance of a thymus. As in the intestinal epithelium, RA and RARs are important regulators of thymic epithelial cell homeostasis, and thymic mesenchymal cells are a major source of RA during embryogenesis (45). Furthermore, in developing thymocytes, ligation of RARγ/RXR heterodimers by atRA or 9-cis- RA leads to glucocorticoid-mediated cell death involved in apoptosis by neglect of double-positive (DP) thymocytes. Hence, in vivo treatment with RARγ agonists results in thymic involution due to massive death of DP thymocytes (46, 47). Activation of RARα by large doses of atRA can override RARγ-induced death, whereas strong costimulation of RXR by 9-cis-RA neutralizes the inhibitory effect of RARα (46)."
"Although critical for embryonic development and lymphopoiesis, RA and RARs are first and foremost effective at controlling postnatal immune functions, especially at the mucosal border of the intestine. Flexibility to balance between optimal protective immunity and effective peripheral tolerance is a prerequisite for the mucosal immune system that operates at this largest interface of the body and is thus exposed to numerous pathogen-derived antigens but at the same time is continuously exposed to a vast load of antigens from the diet and commensals that need to be tolerated. Aberrant immune responses directed towards such innocuous antigens can cause local chronic inflammation that often spreads systemically. Consequently, optimal protective immunity has to coincide with effective immune regulation, and RA and its receptors play critical roles in maintaining this balance. Although RA is typically associated with immunosuppressive roles, RA and RARs can also function as initiators of inflammatory responses and protective immunity. Because of this flexibility, these molecules are ideal to control mucosal immune responses and immunity in general; therefore, just about every immune cell is profoundly affected in one way or the other by the signals and molecular mechanisms controlled by RA and its receptors."
"Besides inducing RALDH2 expression, RA influences the maturation and antigen-presentation function of DCs and their ability to prime T cells in either a tolerogenic or an inflammatory fashion."
"Paradoxically, although [] RA-based regulatory functions are all critical to maintain immune homeostasis and warrant steady state immune tolerance, RA also mediates proinflammatory effects on DCs and promotes proinflammatory priming of T cells during an infection or under inflammatory conditions. Furthermore, instead of promoting apoptosis of antigen-presenting DCs, RAR/RXR signaling in conjunction with inflammatory stimuli can lead to survival and maturation of inflammatory DCs, which then upregulate their production of RA in a feedback-loop system and promote increased RA signaling at sites of inflammation (72). RA can further cooperate with inflammatory cytokines such as IL-15 to enhance the production of IL-12p70 and IL-23 by DCs (73) (Figure 3)."
"The effect of RA on macrophages, similar to its effect on DCs, can be anti- or proinflammatory. Upon LPS stimulation, for example, RA decreases the production of IL-12, TNF-α, nitric oxide (NO), PGE2, or COX2 (75–78), whereas it enhances the production of IL-10 (77). Furthermore, IL-10 can feed back on the macrophages and further limit their production of proinflammatory cytokines (79). Alternatively, RA can cooperate with GM-CSF to promote the phagocytic antigen presentation function of macrophages leading to induced regulatory T cell (Treg) formation and immune tolerance (65). In contrast, ligation of RARγ in macrophages controls optimal production of inflammatory cytokines in response to microbial stimuli resulting in a more effective pathogen control, as observed in a Mycobacterium tuberculosis infection (80). Overall, these observations indicate that RA and RAR signaling in mucosal APCs greatly affects the course of the immuneresponse, underscoring the central role of RA in controlling the balance between mucosal immunetolerance and protective immunity."
"The effects of RA signaling in T cells, similar to their effects on the APCs, are context dependent and can be promoting or inhibiting depending on the local and/or intracellular dose of RA, the expression of specific RAR isoforms, the specific cytokine environment, and the nature of the responding T cell."
"Despite its role in the reciprocal regulation of enhanced iTreg generation but suppressed Th17 cell differentiation, RA at low doses is also essential for the functional differentiation of effector T cells. In addition to impaired homing to the site of inflammation, differentiation of effector CD4 T cells is also defective in VAD mice or mice deficient for RARα (43). Furthermore, IFN-γ and IL-17A production by Th1 and Th17 cells in response to an infection was reduced in the absence of RA signaling in T cells (43). Similar results were observed in mouse models of vaccination or allogeneic graft rejection when transcriptional activity of RARs was specifically ablated in CD4 T cells (106). Although linked to the pathogenesis of autoimmune and excessive inflammatory responses, Th17 cells are equally important for protective immunity at the mucosal interface of the intestine, and they are typically present at steady state in this compartment, but they are virtually ablated in VAD mice (43). The ability of APCs to produce IL-6 is also reduced in VAD mice (43), and in a proinflammatory context involving cytokines, such as IL-15, RA acts through DCs to block conversion into Tregs and to promote Th1 effector polarization (73). Therefore, the ability of RA to support effector differentiation probably results from combined actions on DCs and T cells, because addition of RA to Th17 cell–polarizing conditions in APC-less cultures did not enhance effector polarization (107)."
"[..]although mucus plays a critical role in protecting the intestinal barrier, vitamin A has a negative effect on mucus production by goblet cells, which conversely increased under VAD conditions (160). [¿] Enhanced mucus production is further promoted by IL-13 produced by the expanded ILC2 population in the absence of vitamin A (160). Therefore, although an insufficiency of vitamin A greatly impairs the adaptive Th2 response, it equally increases the passive barrier protection and the innate ILC2 response and thus maintains or even increases control of nematode infections (160) (Figure 3). The balanced compensation of RA/RAR signaling in the innate and adaptive immune system allow for flexibility and warrant effective protective immunity adjustable to the circumstances or environmental triggers."
"Despite the regulatory properties of RA mentioned [], the action of RA produced by [] different cell types in the gut strongly depends on the physiopathological context []. For example, a study conducted with samples from patients with Crohn’s disease, a very severe inflammatory bowel disease, reported that RAR signalling promotes the differentiation of TNFα-producing inflammatory macrophages and that macrophages from the intestinal mucosa of Crohn’s disease patients are more potent to produce RA (Sanders et al., 2014)."
"Vitamin A and RA promote the differentiation of CD4 helper T cells towards the Th2 phenotype over Th1. Consequently, high-level dietary vitamin A enhanced and VAD diet reduced the development of experimental asthma in a mouse model (Schuster et al., 2008). Although this effect of RA seems to be mainly indirect, via its action on DCs, the use of specific agonists and antagonists revealed that RA, via RARα, directly suppresses Th1 development and enhances Th2 differentiation in murine (Iwata et al., 2003) and human CD4 T cells (Dawson et al., 2008)."
"Although the reciprocal role of RA on Treg and Th17 cell differentiation has been extensively studied, the effect of RA on Th17 differentiation or on already differentiated Th17 seems to depend on the available concentration of RA in the milieu, as well as on the inflammatory/steady-state context. Indeed, several studies have reported that RA could promote Th17 differentiation while in other reports, RA treatment significantly reduced the number of Th17 (Larange and Cheroutre, 2016). The available concentration of RA, the induction of gut homing receptors on Th17 cells and the environmental context could explain these discrepancies (Takahashi et al., 2012; Wang et al., 2010). RA and RARα also play a central role in the phenotype maintenance of already differentiated Th1 cells, by repressing the Th17 differentiation program (Brown et al., 2015) (Figure 4)."
"In addition to its broad ability to confer gut tropism, RA exerts multiple and sometimes paradoxical functions on virtually all types of immune cells, placing this nutrient-derived factor as a central regulator of the immune response. Unlike some unilaterally decisive factors that will systematically steer the immune response in one direction, RA acts more as an adjuvant that will regulate the immune system depending on the physiopathological context, sometimes pushing the balance towards the regulatory side and sometimes towards the inflammatory side. The proper action of RA is guaranteed by the tight regulation of RA metabolism (both organ and cell-type dependent), as well as by the multiplicity of mechanisms of action of RARs, that in addition to their transcriptional functions interfere with many signalling pathways in the nuclear and cytosolic compartments of a cell."
A different version of the previous figure.
View attachment 12363
Blossom, it's possible that your experiencing some of these when you ingest plenty of poison/"vitamin" A.
- Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells (check out figures)
"Retinoic acid (RA), the main biologically active metabolite of vitamin A, has been shown to modulate responses of various immune cells [10]. For example, RA enhances expression of the gut-homing integrin a4ß7 in both murine and human B cells and CD4+ T cells [11–13]. Moreover, RA cooperates with TGF-ß to promote the conversion of naive CD4+ T cells into Foxp3+ Treg cells in mice as well as humans [14, 15]. In contrast, RA has been shown to activate effector CD4+ T cells under proinflammatory conditions. The RA-retinoic acid receptor a (RARa) axis is essential for the production of the proinflammatory cytokines IFN-y and IL-17A by Th1 and Th17 cells in response to infection [16]. Furthermore, RA enhances Th2 responses in human CD4+ T cells in vitro and in helminth-infected mice [12, 17–19]. A few recent studies have reported an effect of RA on murine ILCs. Mielke et al. showed that RA promotes production of IL-22 in ILC3 cells stimulated with IL-1ß and IL-23 [20]. In addition, Van de Pavert et al. observed that fetal RA signaling controls the differentiation of LTi cells, which are a subset of ILC3 cells and crucial for the formation of secondary lymphoid organs. The authors established that maternal levels of dietary vitamin A control the size of secondary lymphoid organs and the efficiency of immune responses in the adult offspring [21]."
"In the present study, we hypothesized that RA and 1,25D3 influence human ILC responses. Because our primary interest is in allergy, we put emphasis on ILC2 cells. We observed that RA acts synergistically with IL-2 and other cytokines known to stimulate ILCs, to induce expression of the gut-homing integrin a4ß7, as well as production of IL-5 and IL-13 in ILC2 cells, and IFN-y in ILC1 and ILC3 cells. In contrast, 1,25D3 inhibited the effects of RA on cytokine production and expression of gut-homing integrin. Together, these data shed a light on the effect of two immunomodulatory vitamins on human ILC responses. The balance between these vitamins, which has shifted with industrialization, may be an important factor in the development of allergic inflammation and other chronic immune disorders in which ILCs play a role."
"Allergic and other chronic immune-mediated diseases have become rapidly more prevalent in association with modernization and urbanization around the world [29–31]. There are myriad environmental changes suggested to partially explain this association. The ‘vitamin hypothesis’ posits that nutritional shifts accompanying these lifestyle changes, including increasing vitamin A sufficiency and vitamin D deficiency, may contribute to this trend by influencing conventional CD4+ T cell, B cell and basophil responses [22, 24, 32]. Our findings presented here suggest that ILCs may also be influenced in a pro-inflammatory manner by those changes."
"AD [Atopic Dermatitis] is often the first manifestation of the ‘atopic march’, and approximately one-third of young children with moderate-to-severe AD develop food allergies, which are frequently participating triggers of the inflammation [37]. An important hallmark of AD is a defective epidermal barrier function, which allows the penetration of environmental triggers such as allergens, and their interaction with the immune system."
"We observed that 1,25D3 inhibits RA-induced production of effector cytokines and expression of gut-homing integrin in human ILCs. ILC2 cells have been shown to enhance allergic inflammation, and two recent studies reported that ILC2 cells also facilitate sensitization to allergens [6, 35, 42, 45–47]. Inhibition of ILC2 cell activation and gut homing by 1,25D3 could therefore ameliorate allergic inflammation, particularly in the gastro-intestinal tract, and perhaps in this way play a role in prevention of allergic disease."
"In summary, we have shown that the biologically active metabolites of vitamins A and D have profound effects on cytokine production and homing of human ILCs. Our data provide additional evidence for a mechanism by which alterations in nutrition and exposure to sunlight may influence immunity."
- Retinoic Acid and Its Role in Modulating Intestinal Innate Immunity
"Given the recent findings on the role of VA affecting both the innate and adaptive arms of the immune system, it seems plausible to implicate VA deficiency in increased susceptibility towards infectious diseases [7,8]. On the other hand, VA excess leads to deregulation of liver metabolic functions and presents several other toxic effects [9]. The role of VA metabolite atRA in adaptive immune responses has been extensively reviewed elsewhere [10,11,12], particularly in the context of intestinal immunity. Here, we review the recent findings where atRA plays a central role in the development and functioning of the innate arm of the immune system, in particular the myeloid compartment and innate lymphoid cells (ILCs)."
"Besides being the primary site for VA absorption, intestinal epithelial cells (IECs) can metabolize VA to RA by their ability to express ALDH1A1 [19,20]."
"After differentiation and migration to the intestine, specific DC subsets are endowed with the ability to sense and respond to atRA [45]. The general consensus on the effect of atRA on DC function is to promote an anti-inflammatory phenotype characteristic of intestinal DCs [46,47]. Transcriptional profiling of in vitro differentiated DCs in the presence of atRA showed downregulation of pro-inflammatory genes involved in the NF-kB mediated inflammatory program [45]. However, besides the above-mentioned immuno-regulatory effects, some studies have emphasized a rather unorthodox pro-inflammatory role of atRA in DCs. In the presence of IL-15, atRA was shown to act as an adjuvant in promoting the secretion of the pro-inflammatory cytokines IL-12 and IL-23 by DCs [48]. Similarly, human DCs generated from monocytes in the presence of atRA primarily induced interferon gamma (IFNy) production by CD4+ T cells in vitro [49]. The dual role of atRA in influencing DCs function might be a result of the microenvironments and/or cytokine milieu to which the DCs are exposed. For example, atRA in a cytokine environment that is either pro- or anti-inflammatory would induce a tolerogenic or pro-inflammatory DC phenotype, respectively."
"Besides being able to sense and get influenced by atRA, DCs, in turn, are one of the main producers of atRA, a phenomenon that has been well-studied in intestinal CD103+ DCs expressing high levels of the Aldh1a2 gene. This is most evident in the intestine where CD103+ DCs in the proximal tract of the small intestine are exposed to higher levels of atRA, which, in turn, renders them better atRA producers compared to distal small intestinal or colonic DCs. However, this is not limited to the intestine since extra intestinal DCs endowed with the ability to produce atRA have also been described, such as CD103- DCs in the skin [41]. Importantly, CD103- DCs in the skin can induce Foxp3+ TREG similar to intestinal CD103+ DCs [41]. Several factors can influence DCs into atRA-producing cells. For example, incubation of DCs with atRA alone or in combination with various cytokines such as interleukin-4 (IL-4) and transforming growth factor beta (TGFß) cytokines were able to induce Aldh1a2 mRNA expression, which seems to be sufficient to render DCs with the capacity to metabolize atRA [50,51,52]. In addition, the short chain fatty acid butyrate was shown to induce Aldh1a expression in monocyte-derived dendritic cells, suggesting that microbiota-derived metabolites might play important roles in intestinal DC function [53]. Moreover, the Wnt pathway seems to be crucial in imprinting intestinal DCs since this pathway seems to be crucial to induce Aldh1a2 expression and produce atRA [54]. Furthermore, MyD88 signaling and, in particular, toll-like receptor (TLR) 1/2 stimulation can also induce Aldh1a2 mRNA expression and imprint extra-intestinal DCs with atRA producing capacity [15,55,56]. While multiple factors and pathways have been shown to influence the atRA producing capacity of DCs, whether two or more of these pathways interact with each other needs to be investigated. Irrespective of the mechanism, DCs imprinted to produce atRA primarily induce a tolerogenic program by generation of Foxp3 TREG cells. Importantly, atRA production by intestinal CD103+ DCs seems to be crucial for the generation of TREG and for the establishment of tolerance towards innocuous dietary antigens, a process known as oral tolerance."
"Local and systemic immune unresponsiveness towards antigens that have been previously administered by oral route is classically defined as oral tolerance [12,57]. These antigens, which are considered innocuous, are primarily derived from diet and failure to mount an effective tolerance towards these antigens has been associated with conditions such as food allergies and celiac disease. VA has proven crucial to the establishment of oral immunological tolerance against food antigens [12,57], and its deficiency might contribute towards food allergies, celiac disease [¿] and inflammatory bowel diseases (IBD). Indeed, mice deprived of VA resulted in exacerbated intestinal inflammation as assessed by colitis score and colon length using the dextran sodium sulfate (DSS)-induced colitis model [58]. Moreover, atRA supplementation efficiently attenuates chronic inflammation in a mouse model of ileitis [59]. Notably, atRA participates in more than one of the five-step models proposed for the establishment of oral immunological tolerance [12] (Figure 2)."
"It is well established that the concentrations of atRA in the small intestine follows a proximal (i.e., duodenum) to distal (i.e., colon) decreasing gradient [15,23]. Similarly, ILC subsets are differentially distributed along the gastrointestinal tract with ILC3 outnumbering ILC1 and ILC2 in the small intestine, whereas ILC2 are predominant in the colon [78]. Therefore, it is tempting to speculate that atRA might be one of the players involved in the complex functional regionalization of ILCs in the intestine, likely by controlling their migration, differentiation and/or function."
"Recent studies support the role of atRA as an important enhancer of DC differentiation and migration from the bone marrow to the intestine, ultimately resulting in DCs that are capable of both sensing and producing atRA. In addition, atRA works as a crucial regulator of DC function, which dictates T helper and effector cell function in the mucosal sites and in peripheral tissues. Interestingly, the cytokine milieu can influence DCs to induce pro-inflammatory T helper functions, even in the presence of atRA. Finally, recent advances in the ILC field point towards atRA-producing DCs as modulators of ILC migration, function and phenotype plasticity. Taken together, atRA exerts a crucial role in DC function in order to maintain tolerance against food and microbial antigens and promote tissue homeostasis."
- Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases
"[..]after the absorption and metabolization of vitamin A into RA in the gut, RA plays critical roles in the mucosal immune response as a regulatory signal in the intestinal mucosa by promoting Foxp3 regulatory T cell differentiation [6] and immunoglobulin (Ig) A production [7]. In addition, RA induces the homing of innate immune cells, such as innate lymphoid cells (ILCs) [8] besides regulatory and effector T and B cells, to the gut [9–11]. During infections, RA can induce the production of proinflammatory cytokines by dendritic cells (DCs), promoting the differentiation of effector T cells and the protection of the mucosa [12]. Thus, RA is crucial for maintaining homeostasis at the intestinal barrier and equilibrating immunity and tolerance. Due to the extensive role of RA in immune cells and the immune response, reducing mortality in children by vitamin A supplementation may be possible [4]."
"In addition, due to its regulatory activity, RA has been shown to play an important role in the control of inflammatory diseases not only in the intestine [13, 14] but also in other tissues, such as the central nervous system [15–17] and pulmonary mucosa [18, 19]."
"After uptake, RA is generated from retinol by two sequential reactions. In the first reversible reaction, retinol is oxidized into retinal by the ubiquitously expressed enzyme alcohol dehydrogenase (ADH) [20]. Subsequently, in intestinal epithelium cells, DCs and macrophages associated with mesenteric lymph nodes (mLNs) and Peyer’s patches (PPs), retinal is oxidized by the enzyme retinal dehydrogenase (RALDH) to generate RA [1]. There are three isoforms of RALDH (RALDH1, RALDH2, and RALDH3) [21], and [again,] their expression is tightly regulated and limited in the cells mentioned above. Thus, RALDH is considered the main enzyme that defines the populations of cells that are capable of producing RA [20]. Intestinal epithelium cells can also metabolize vitamin A after absorption into retinal and RA, which can be directly released into the intestinal mucosa [21]."
"RA can be generated in multiple forms as all-trans, 9-cis, and 13-cis RA [26, 27]; however, all-trans RA (atRA) is physiologically the most abundant [28]. RA interacts with nuclear receptors, such as the retinoic acid receptor (RAR) and retinoid receptor X (RXR), to regulate the transcription of several target genes [10, 29] by binding the retinoic acid-responsive elements (RAREs) in DNA [30]. These receptors form heterodimers; RAR comprises three major isoforms (a, b, and y) that interact with all forms of RA, whereas RXR, which also has the a, b, and y isoforms, mainly interacts with 9-cis RA [31]. RA can also signal through peroxisome proliferator-activating receptor beta (PPAR-ß) when it forms a heterodimer with RXR, which may be important for lipid metabolism and glucose homeostasis [1]."
"Control of the RA concentration in tissues is performed by a group of enzymes that belong to the cytochrome P450 family 26 (CYP26), including subfamilies A1, B1, and C1 (CYP26A1, CYP26B1, and CYP26C1), which catalyze RA present in the cytosol to generate the oxidized forms (5,8-epoxy RA, 4-oxo RA, 4-hydroxy RA, and 18-hydroxy RA) [34, 35]. The action of these enzymes prevents RA accumulation in the organism and maintains optimal physiological RA concentrations for the best performance."
"Some inflammatory factors may influence RALDH expression, such as prostaglandin E2 (PGE2), which is produced by peripheral stromal cells and suppresses the differentiation of RA+ DCs by directly antagonizing RALDH expression [50]. In addition, DCs that infiltrate the gut during inflammation do not acquire RALDH activity, which is required for RA synthesis. These inflammatory DCs express E-cadherin and the CD103 receptor, accumulate in the mesenteric lymph nodes and the inflamed colon, exhibit high expression of Toll-like receptors (TLRs) and produce cytokines IL-6 and IL-23, enhancing inflammation [47]."
"In infections, RA signaling may also induce the production of proinflammatory cytokines by DCs, promoting the differentiation of effector T cells [12] and enhancing the cellular activation state, in addition to the promotion of the formation of tertiary lymphoid structures [10]. These structures are formed in response to nonresolving inflammation generating lymphoid aggregates that drive adaptive immune reactions [54, 55]. RA also influences the maturation of monocyte-derived DCs (MoDCs) by increasing the expression of major histocompatibility complex (MHC) class II and CD86 and regulating the survival of DCs via the RARa/RXR pathway [56]. In parallel to the activation of the innate immune response, RA promotes human DCs to induce IL-10-producing T cells to control inflammation and the maintenance of tissue homeostasis [57]."
- Retinoic Acid, Leaky Gut, and Autoimmune Diseases (@Tarmander - 3.1/4.3.1)
- Retinoic acid syndrome: a review (massive amounts given in cancre, perhaps there's something to learn from it)
- Vitamin A-Deficient Hosts Become Nonsymptomatic Reservoirs of Escherichia coli-Like Enteric Infections
--
Complementing post #1693..
- Inflamed About Retinoic Acid
↳ Context is key in the gut (don't be fooled by the title)
- Reciprocal TH17 and Regulatory T Cell Differentiation Mediated by Retinoic Acid
- Paradoxical Effects of All-Trans-Retinoic Acid on Lupus-Like Disease in the MRL/lpr Mouse Model
--
Propoison A:
- Concentrations of Selected Carotenoids and Vitamin A in Human Liver, Kidney and Lung Tissue
- Mammalian carotenoid absorption and metabolism
"Carotenoids are ubiquitous in mammalian tissues and the accumulation of carotenoids among animals is species specific and highly variable [64]. These differences could be explained by dietary intake and/or species differences in absorption and metabolism, such as gut motility or handling of lipoproteins by the body. Goodwin [65] classified groups of animals into carotenoid accumulators, non-accumulators, and intermediates. Primates, similarly to humans, accumulate a wide range of carotenoids in high concentrations, while other mammalian species accumulate only one specific carotenoid/class of carotenoid or no carotenoids at all. Birds preferentially accumulate xanthophylls in most tissues [64]. Contrary to past studies, Chew et al. [66] recently reported that the domestic cat absorbs significant amounts of b-carotene from dietary sources. Rats, gerbils, ferrets, and preruminant calves absorb and/or accumulate carotenoids in tissues to varying degrees and thus are animal models used to study absorption and metabolism [67,68]."
[68] Review of Animal Models in Carotenoid Research
- Carotenoids: Linking Chemistry, Absorption, and Metabolism to Potential Roles in Human Health and Disease
"Once in the blood, carotenoids are passively taken up into various tissues following degradation of lipoproteins by lipoprotein lipase (LPL; see Fig. 5). Chylomicron remnants are cleared from the blood in the liver by the chylomicron receptor. Carotenoids accumulate in the liver, but regulation of their storage is poorly understood. Recently, Rao and co-workers (4) purified a cellular carotenoid-binding protein (CCBP) from ferret liver, which had a high degree of specificity toward carotenoids with at least one β-ionone ring, but not toward other carotenoids. This group proposed that CCBP may play a role in storage, transport, and metabolism of provitamin A carotenoids, as well as act as a natural substrate for metabolic reactions involving these carotenoids (4). Carotenoids may exit the liver into the blood following incorporation into very low-density lipoproteins (VLDL). Subsequent uptake of carotenoids into tissues from VLDL, and especially LDL, is thought to occur through the LDL receptor because the tissues with the highest levels of carotenoids (e.g., liver, adrenal glands, testes) tend to have high LDL receptor activity (128,137,138)."
"Carotenoids are present in many tissues in humans, including liver, adipose, pancreas, kidney, lung, adrenal, spleen, heart, thyroid, testes, ovary (87,139), and eye (140–142). Total quantitative levels of carotenoids are highest in liver and adipose tissue, the main storage sites for carotenoids (128). Concentrations of carotenoids (i.e., per gram of tissue basis) are highest in the liver, adrenal, and reproductive tissues. As is true for serum, β-carotene, lycopene, lutein, α-carotene, zeaxanthin, and cryptoxanthin are the main tissue carotenoids (137,139). In addition, the geometrical isomers of lycopene and β-carotene found in serum are also found in tissues (87). Some tissues exhibit specific patterns of carotenoid accumulation, suggesting that certain carotenoids may exert a biological effect in one tissue over another."
- Evidence for compartmentalization of mammalian carotenoid metabolism
- β-Carotene conversion products and their effects on adipose tissue
- A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries)
- Presence of fed β-carotene in digesta, excreta, blood, andhepatic and adipose tissues of Holstein steers
- Carotenoid trade-off between parasitic resistance and sexual display: an experimental study in the blackbird (Turdus merula) (it's cool)
That's understandable.
I'm counting on you US and A citizens to start a chain reaction by requesting from your local producers 'The Return of White Carrots'. They should be featured on your Instagram pages as part of artificial dishes, which in turn will influence artificial people from unimportant countries to copy what you're doing, which in turn will lead to improvised pictures using radishes but at the same time create multiple local demands for producers to grow more of the real deal, and we'll conclude the reactions with a comment with hints of hypotyphoid selfishness that it will eventually benefit me for making it easier to buy them. Thank you as well in advance.
Brother John
Member
- Joined
- Feb 10, 2016
- Messages
- 101
Blossem that is a great list. Mine is similar for the results I have from extremely v A intake. Janell is right we need a "results threat" .Here is mine that I can think of at the moment:
Skin-resolved redness, sensitivity.
Nails -beau’s lines growing out.
Hair-smoother, less shedding.
Energy-can work 12 hours on my feet in critical care easily now.
Mood-no depression/sad/irritability.
Bowels-regular 2x per day.
Hormones-menopause symptoms and thyroid significantly improved.
Sleep -uninterrupted most nights-I also sleep inclined.
Inflammation- seem to have none remaining.
Joint pain- hip, knees and neck/shoulder pain resolved.
Edema-mainly in upper arms is gone.
Blood pressure (started at 135/90 and now 110/75)
Cholesterol greatly improved (total from 260 down to 180)
Tolerate more foods without any nausea, cramping.
Memory/cognitive function improved- passed state boards on the first try (test has a 50% pass rate)
Vision clearer consistently- I still use reading glasses though.
More resilient to stress- mental and physical. I can go without eating for many hours if necessary without getting weak. Things that would have felt overwhelming before do not impact me nearly as intensely.
Resolved hirsutism- although probably a mild-moderate case I still didn't like it at all. I now have less than 5 softer lighter hairs that grow gradually and are easily managed versus having to inspect my chin and upper lip for new thick dark (manly) hairs multiple times daily.
Thanks,
Brother John
That's wonderful, I'm very happy for you @Brother John.Blossem that is a great list. Mine is similar for the results I have from extremely v A intake. Janell is right we need a "results threat" .
Thanks,
Brother John
Brother John
Member
- Joined
- Feb 10, 2016
- Messages
- 101
Thanks Blossom and same for you!That's wonderful, I'm very happy for you @Brother John.
I have been working a Lot and not reading this thread for a couple weeks and I just read over 20 pages, well sort of read... I must say I skipped a lot. I have a comment or two: We all, like it or not, are experiments of life. When people advocate against experimenting with diet, supplements, detox etc based on studies with walls of text included, I am amazed. It as though some think the more words about a subject the more truth is contained. I live in my body. I and it are one. I experiment all the time in every aspect of my life. I don't want a "guaranteed outcome" from anyone because that guarantee is worth nothing.... So I don't look for guarantees. I look for ideas that seem worth experimenting with... And truth be told, sometimes I go too far to find out how far too far is. And haven't you? Even if that was not your intention??
I am eating a very low A diet and have been for app 6 months. I eat rice and beef mostly. I am doing better with this than I did with fruit and high A foods.
Before I do an experiment I do look at risk factors and keep track of negative and positive outcomes...
I encourage everyone to experiment consciously .... because you are already experimenting with your health anyway! I don't care to add up all the research pro A and all the research anti A.... I would rather cut to the chase and do the experiment. I do this for me. Anyone objects, too bad..
I thank Grant Generaux for taking the time and courage to do what so many would say is "wrong".... In closing: Just because we have mountains of data regarding human health... we still don't know how the miracle of a life for works....
Thanks,
Brother John
Beautiful and well said!Thanks Blossom and same for you!
I have been working a Lot and not reading this thread for a couple weeks and I just read over 20 pages, well sort of read... I must say I skipped a lot. I have a comment or two: We all, like it or not, are experiments of life. When people advocate against experimenting with diet, supplements, detox etc based on studies with walls of text included, I am amazed. It as though some think the more words about a subject the more truth is contained. I live in my body. I and it are one. I experiment all the time in every aspect of my life. I don't want a "guaranteed outcome" from anyone because that guarantee is worth nothing.... So I don't look for guarantees. I look for ideas that seem worth experimenting with... And truth be told, sometimes I go too far to find out how far too far is. And haven't you? Even if that was not your intention??
I am eating a very low A diet and have been for app 6 months. I eat rice and beef mostly. I am doing better with this than I did with fruit and high A foods.
Before I do an experiment I do look at risk factors and keep track of negative and positive outcomes...
I encourage everyone to experiment consciously .... because you are already experimenting with your health anyway! I don't care to add up all the research pro A and all the research anti A.... I would rather cut to the chase and do the experiment. I do this for me. Anyone objects, too bad..
I thank Grant Generaux for taking the time and courage to do what so many would say is "wrong".... In closing: Just because we have mountains of data regarding human health... we still don't know how the miracle of a life for works....
Thanks,
Brother John
I'm getting my vitamin A blood test done in the morning and it will be interesting to see the results. I'm on day 225 of low A.
Somewhat sadly I must admit that I do not trust science much anymore. I'm sure there are some good scientist just as there are some good doctors but I think there's plenty of greed and fraud going on behind-the-scenes.
Like you I'm sticking with my own experience on this one for now.
InChristAlone
Member
Great! Way to 'perceive, think, act'!Thanks Blossom and same for you!
I have been working a Lot and not reading this thread for a couple weeks and I just read over 20 pages, well sort of read... I must say I skipped a lot. I have a comment or two: We all, like it or not, are experiments of life. When people advocate against experimenting with diet, supplements, detox etc based on studies with walls of text included, I am amazed. It as though some think the more words about a subject the more truth is contained. I live in my body. I and it are one. I experiment all the time in every aspect of my life. I don't want a "guaranteed outcome" from anyone because that guarantee is worth nothing.... So I don't look for guarantees. I look for ideas that seem worth experimenting with... And truth be told, sometimes I go too far to find out how far too far is. And haven't you? Even if that was not your intention??
I am eating a very low A diet and have been for app 6 months. I eat rice and beef mostly. I am doing better with this than I did with fruit and high A foods.
Before I do an experiment I do look at risk factors and keep track of negative and positive outcomes...
I encourage everyone to experiment consciously .... because you are already experimenting with your health anyway! I don't care to add up all the research pro A and all the research anti A.... I would rather cut to the chase and do the experiment. I do this for me. Anyone objects, too bad..
I thank Grant Generaux for taking the time and courage to do what so many would say is "wrong".... In closing: Just because we have mountains of data regarding human health... we still don't know how the miracle of a life for works....
Thanks,
Brother John
Truth right there. Beautiful.Thanks Blossom and same for you!
I have been working a Lot and not reading this thread for a couple weeks and I just read over 20 pages, well sort of read... I must say I skipped a lot. I have a comment or two: We all, like it or not, are experiments of life. When people advocate against experimenting with diet, supplements, detox etc based on studies with walls of text included, I am amazed. It as though some think the more words about a subject the more truth is contained. I live in my body. I and it are one. I experiment all the time in every aspect of my life. I don't want a "guaranteed outcome" from anyone because that guarantee is worth nothing.... So I don't look for guarantees. I look for ideas that seem worth experimenting with... And truth be told, sometimes I go too far to find out how far too far is. And haven't you? Even if that was not your intention??
I am eating a very low A diet and have been for app 6 months. I eat rice and beef mostly. I am doing better with this than I did with fruit and high A foods.
Before I do an experiment I do look at risk factors and keep track of negative and positive outcomes...
I encourage everyone to experiment consciously .... because you are already experimenting with your health anyway! I don't care to add up all the research pro A and all the research anti A.... I would rather cut to the chase and do the experiment. I do this for me. Anyone objects, too bad..
I thank Grant Generaux for taking the time and courage to do what so many would say is "wrong".... In closing: Just because we have mountains of data regarding human health... we still don't know how the miracle of a life for works....
Thanks,
Brother John
Way to 'perceive, think, act'!

Amazoniac
Member
Why did you prefer to go to the cold turkey instead of removing each form separately? Starting for example by minimizing propoisons gradually until exclusion, and only then moving to preformed if desired. It's something for others to consider, it might help in grasping what's going on.So It's been a week since I've adopted a very-low vitamin A diet. I have stopped my previously ray peat diet, and my diet is:
-White rice
-Beef (200 gr a day)
-5 egg whites
- Coffee with a lot of sugar and coke
What I've noticed so far:
- Eczema, no improvement (yet)
- Asthma, much improvement!
- Well being, much improvement. My mind is much clearer. I feel less brain fogged.
I feel quite good on this diet. I am going to continue for sometime to see if I can cure my eczema.
I don't know if my intake of Vitamin A is zero though, because I just buy beef from the local supermarket and its grass-fed (irish-beef) instead of grain-fed.
Tarmander, you must've not read it all..re the leaky gut study you showed me. If you were following Dr. Smith's rules, when a study shows something is improved by A in the short term, that thing gets totally trashed in the long term. So if RA can help leaky gut in the short term, in the long term....well I would say my experience of allergies and leaky gut matches this pretty well.
Last edited:
schultz
Member
- Joined
- Jul 29, 2014
- Messages
- 2,653
@Blossom You said OJ has vit A? hmm this is interesting.. I don't like OJ that much.. The taste of liver in general disgusts me. Eggs give me bloating problems. Could I be doing low vitamin A diet unintentionally? Two weeks ago, I asked myself "when was I the last time I had liver?" and the answer to my surprise was more than 2 months ago. So I took myself to the restaurant and ordered two grilled lamb liver sandwiches and I had the worst migraine the following day. I was also anti-social when I went for my family lunch gathering. This theory could have some truth even though I don't want to jump to conclusions and I strongly advise people to test the theory by themselves. What do you think of vitamin D supplementation? @Janelle525 do you take vitamin D?
Orange Juice has beta-carotene.
Ray has talked about how eating liver can temporarily make you hypothyroid. He actually said "two big meals of liver" when he was talking about it. Certain amino acids in muscle meat and liver are also thyroid suppressive.
"RAY PEAT: Well, liver is another suppressive food. If you eat two big meals of liver, you can see a big decrease in thyroid function. JOHN BURKHAUSEN: And how long does that suppression last from liver or cabbage? If you eat something and – say, you're not hyperthyroid, you’re just normal, whatever that is, and you eat some liver, how long does it take for your thyroid to bounce back? Is that also just a matter of a couple of days? RAY PEAT: Yeah. "
Long-term VAD decreased neurogenesis and led to memory deficits. More importantly, these effects were reversed by 4 weeks of RA treatment.
I suppose this is what you'd expect when you restrict a nutrient needed for making steroid hormones, some of which are brain protective.
Ray said this in a podcast...
"Yeah, vitamin A besides being a matter of sensitivity to electronic excitations such as in the eye and acting as sort of as an antenna for interacting with electromagnetic energy - the other region of vitamin A activity is in activating the enzymes that makes steroids and especially brain steroids. So if your vitamin A is deficient, you not only have poor vision and skin problems and so on, but your ability to make pregnenolone, progesterone, DHEA and their derivatives is limited and the brain is the major steroid-forming organ, the skin, the gonads, the adrenals and the brain. The brain is probably the most powerful steroid forming organ when it’s working right and vitamin A is essential for making those."
If I understand correctly VA isn’t stored in muscle tissue but can be found in the fat. If the fat is white it shouldn’t have much VA or carotenes.
I think vitamin A is colourless. Things like goat milk and buffalo milk have low beta-carotene and will make white butter, even though the vitamin A is present. When I had chickens I would feed them a lot of liver, and if they didn't have access to the outdoors (if it was winter for example) their yolks would be incredibly pale. I've never seen egg yolks so pale before. I only fed them potatoes and liver so they had no beta-carotene in their diet. The yolks were almost white.
EMF Mitigation - Flush Niacin - Big 5 Minerals
Similar threads
- Replies
- 24
- Views
- 9K
Low Toxin Videos
Grant Genereaux on Vitamin A, retinol and retinoic acid Toxicity...
- Replies
- 2
- Views
- 1K
Low Toxin Diet
Simple steps to transition to a Low Toxin Diet/Way of Living
- Replies
- 64
- Views
- 9K
- Replies
- 75
- Views
- 15K
- Replies
- 94
- Views
- 16K
- Replies
- 16
- Views
- 4K
Low Toxin Testimonials
Low Vitamin A Diet Testimonials
- Replies
- 87
- Views
- 23K
- Replies
- 201
- Views
- 24K
- Replies
- 13
- Views
- 7K