According to this study Usual Vitamin Intakes by Mexican Populations, Mexicans consume about 500-600 mcg/d of vitamin A (RAEs). Are you really saying that regular American consumes 5000-6000 mcg ( 16 666- 20 000 IU) of vitamin A per day? How is that possible?Mexicans consume about a tenth of what Americans consume
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
-
By using this site you agree to the terms, rules, and privacy policy.
-
Charlie's Restoration Giveaway #2 (Entire Home EMF Mitigation & Protection Along With Personal Protection) - Click Here To Enter
-
Dear Carnivore Dieters, A Muscle Meat Only Diet is Extremely Healing Because it is a Low "vitamin A" Diet. This is Why it Works so Well...
Rest the rest of this post by clicking here
-
The Forum is transitioning to a subscription-based membership model - Click Here To Read
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Low Toxin Diet Grant Genereux's Theory Of Vitamin A Toxicity
Did a little digging and found that the EAR (estimated average requirement) for vitamin A is 600 mcg for men and 500 mcg for women. So this is the daily intake that you actually need and where virtually nothing gets stored in your body. RDA level is set so high that you get a little vitamin A stored in your liver every day and this allows you to survive months even if you don't get any vitamin A at all.
This is where all the "nutritarian" people go wrong, they think that it's very important to get at least twice the RDA of nuuuuutrients in your body every day because RDA is so small. In truth the RDA is actually pretty high and it guarantees that you don't get any deficiencies at all and you actually accumulate the nutrient in your body.
Mexicans daily intake of A is actually perfect, they get it just the right amount where virtually nothing gets stored and there's no deficiency either. I wish @tim333 would clarify where he found that Mexicans get very little A, because according to my research they get just the right amount.
This is where all the "nutritarian" people go wrong, they think that it's very important to get at least twice the RDA of nuuuuutrients in your body every day because RDA is so small. In truth the RDA is actually pretty high and it guarantees that you don't get any deficiencies at all and you actually accumulate the nutrient in your body.
Mexicans daily intake of A is actually perfect, they get it just the right amount where virtually nothing gets stored and there's no deficiency either. I wish @tim333 would clarify where he found that Mexicans get very little A, because according to my research they get just the right amount.
yep, Ray said he thinks the iron rda should be downgraded to 8mg iirc, and i think in the last couple years that is how the supp companies calculate it, they have 8mg rda iron for men and 18mg for women. zinc rda used to be 15mg, it got downgraded to 11mg. copper used to be 2mg, went down to 0.9mg. chromium went from 120mcg to 35mcg (even the 35mcg is insanely hard to get from diet unless u have lots of raw broccoli or some obscure food items like certain seeds), molybdenum went from 75mcg to 45mcg, manganese i think was 2.3mg down to 2mg, selenium 80mcg or 70mcg to 55mcg. vitamin A itself actually used to have an rda of 5000IU or 1500mcg and went down to 3000IU/900mcg.Did a little digging and found that the EAR (estimated average requirement) for vitamin A is 600 mcg for men and 500 mcg for women. So this is the daily intake that you actually need and where virtually nothing gets stored in your body. RDA level is set so high that you get a little vitamin A stored in your liver every day and this allows you to survive months even if you don't get any vitamin A at all.
This is where all the "nutritarian" people go wrong, they think that it's very important to get at least twice the RDA of nuuuuutrients in your body every day because RDA is so small. In truth the RDA is actually pretty high and it guarantees that you don't get any deficiencies at all and you actually accumulate the nutrient in your body.
Mexicans daily intake of A is actually perfect, they get it just the right amount where virtually nothing gets stored and there's no deficiency either. I wish @tim333 would clarify where he found that Mexicans get very little A, because according to my research they get just the right amount.
but i wonder, if you take in higher amounts vitamin D would it make it safer to eat more vitamin A. cuz if youre eating like an ounce liver a day and half gallon milk a day, your vitamin A is gonna be around 7000IU a day...
meatbag
Member
- Joined
- Jan 15, 2016
- Messages
- 1,771
do you recall where he said the RDA for iron should be 8mg?yep, Ray said he thinks the iron rda should be downgraded to 8mg iirc, and i think in the last couple years that is how the supp companies calculate it, they have 8mg rda iron for men and 18mg for women. zinc rda used to be 15mg, it got downgraded to 11mg. copper used to be 2mg, went down to 0.9mg. chromium went from 120mcg to 35mcg (even the 35mcg is insanely hard to get from diet unless u have lots of raw broccoli or some obscure food items like certain seeds), molybdenum went from 75mcg to 45mcg, manganese i think was 2.3mg down to 2mg, selenium 80mcg or 70mcg to 55mcg. vitamin A itself actually used to have an rda of 5000IU or 1500mcg and went down to 3000IU/900mcg.
but i wonder, if you take in higher amounts vitamin D would it make it safer to eat more vitamin A. cuz if youre eating like an ounce liver a day and half gallon milk a day, your vitamin A is gonna be around 7000IU a day...
I'm so lost, I don't know which way to go. Should I try vitamin A elimination diet or do I go for real animal based vitamin A way?
All of my health problems started when I was at the end of my second heavy accutane course. I started having really bad cranial pressure, my face started to have hot flushes and my back was sweaty all the time even though it was really chilly outside. I also took Ast-ss brand multivitamin which had quite high vitamin A content. I have never really eaten any real animal based vitamin A rich foods, my A intake has come from supplements.
Chris Masterjohn says that accutane actually causes real vitamin A deficiency and EastWest healing says that vitamin A supplements don't work like real animal based A.
So I really don't know what to do, do I have real animal based vitamin A deficiency or do I have toxicity that only goes away with total A elimination. This is so hard and I'm so tired that I don't even know do I have the strength and will to even find out anymore.
All of my health problems started when I was at the end of my second heavy accutane course. I started having really bad cranial pressure, my face started to have hot flushes and my back was sweaty all the time even though it was really chilly outside. I also took Ast-ss brand multivitamin which had quite high vitamin A content. I have never really eaten any real animal based vitamin A rich foods, my A intake has come from supplements.
Chris Masterjohn says that accutane actually causes real vitamin A deficiency and EastWest healing says that vitamin A supplements don't work like real animal based A.
So I really don't know what to do, do I have real animal based vitamin A deficiency or do I have toxicity that only goes away with total A elimination. This is so hard and I'm so tired that I don't even know do I have the strength and will to even find out anymore.
Quelsatron
Member
- Joined
- Jan 1, 2020
- Messages
- 484
Vitamin A is known to lower the blood levels of vitamin D, and i personally experienced getting lots of sunlight last summer but still having 40% of the optimal vitamin D levels in january, after eating liver all autumn. So, I don't know if high D intake/production really protects against vitamin A itself, but it sure protects against it's own vitamin A-induced depletion.yep, Ray said he thinks the iron rda should be downgraded to 8mg iirc, and i think in the last couple years that is how the supp companies calculate it, they have 8mg rda iron for men and 18mg for women. zinc rda used to be 15mg, it got downgraded to 11mg. copper used to be 2mg, went down to 0.9mg. chromium went from 120mcg to 35mcg (even the 35mcg is insanely hard to get from diet unless u have lots of raw broccoli or some obscure food items like certain seeds), molybdenum went from 75mcg to 45mcg, manganese i think was 2.3mg down to 2mg, selenium 80mcg or 70mcg to 55mcg. vitamin A itself actually used to have an rda of 5000IU or 1500mcg and went down to 3000IU/900mcg.
but i wonder, if you take in higher amounts vitamin D would it make it safer to eat more vitamin A. cuz if youre eating like an ounce liver a day and half gallon milk a day, your vitamin A is gonna be around 7000IU a day...
i cant remember where he said 8mg, im actually unsure if he said a specific number now, but back when the rda was 18mg i think he had an article where he said the rda should be revised sharply downward. in the last couple years they have actually set 8mg as the rda for men, while 18mg remains the rda for women. he has said on an all milk diet, youd need to supplement iron, and hes said iron rich blood is fine to supplement if you have a milk diet like the maasai.do you recall where he said the RDA for iron should be 8mg?
meatbag
Member
- Joined
- Jan 15, 2016
- Messages
- 1,771
are you referring to here where he said eggs provide enough iron?i cant remember where he said 8mg, im actually unsure if he said a specific number now, but back when the rda was 18mg i think he had an article where he said the rda should be revised sharply downward. in the last couple years they have actually set 8mg as the rda for men, while 18mg remains the rda for women. he has said on an all milk diet, youd need to supplement iron, and hes said iron rich blood is fine to supplement if you have a milk diet like the maasai.
Ray Peat Email Advice Depository
no, in one of the articles on his site, probably irons dangers, he said he thinks the rda (at the time 18mg) should be revised strictly downward.are you referring to here where he said eggs provide enough iron?
Ray Peat Email Advice Depository
it was a youtube podcast with danny roddy where he mentioned youd need to supplement some iron eventually on an all milk diet, but otherwise milk should provide most of the essential nutrients. when i emailed him asking about blood and the elites drinking blood to potentially stay younger he simply commented "The traditional Maasai diet of iron-deficient milk was supplemented by a small amount of iron-rich blood. Most common diets already have excessive iron."
Amazoniac
Member
- Post-translational protein modification by carotenoid cleavage products
- Vitamin A and D Absorption in Adults with Metabolic Syndrome versus Healthy Controls: A Pilot Study Utilizing Targeted and Untargeted LC-MS Lipidomics
Abstract said:Carotenoids are known to generate various aldehydes, known as carotenoid-derived aldehydes (CDAs), which could efficiently react with protein or DNA. In this in vitro model study, interaction between CDA and protein has been studied. Various proteins were incubated with CDA, and protein modification and adduct formation were confirmed by using matrix-assisted laser desorption and ionization time-of-flight, amino acid analysis, and measuring enzyme activity on modification with CDA. Using radiolabeled NaB((3) H)H(4) and Raney nickel as well as sulfhydryl assay (Ellman's reagent), we confirmed that CDA could conjugate with cysteine through a thioether linkage. The carbonyl assay using 2,4-dinitrophenylhydrazine revealed the possible involvement of Schiff's base reaction between CDA and lysine. The adducts formed between β-apo-8-carotenal (BA8C) and N-acetylcysteine and BA8C and N-acetyllysine were confirmed by HPLC and ESI-MS. Our results suggest that CDA could alter protein function by post-translational interaction with cysteine and lysine by thioether linkage and by schiff's based bonds, respectively. Thus, the formation of CDA adducts with proteins could alter functional properties of proteins responsible for maintaining cell homeostasis and thereby cause cellular toxicity. In view of these observations, further studies are required to understand the delicate balance between beneficial and/or harmful effects of carotenoids as a dietary supplement to slow age-related macular degeneration progression.
- Vitamin A and D Absorption in Adults with Metabolic Syndrome versus Healthy Controls: A Pilot Study Utilizing Targeted and Untargeted LC-MS Lipidomics
Abstract said:Scope:
Persons with metabolic syndrome (MetS) absorb less vitamin E than healthy controls. It is hypothesized that absorption of fat-soluble vitamins (FSV) A and D2 would also decrease with MetS status and that trends would be reflected in lipidomic responses between groups.
Methods and results:
Following soymilk consumption (501 IU vitamin A, 119 IU vitamin D2 ), the triglyceride-rich lipoprotein fractions (TRL) from MetS and healthy subjects (n = 10 age- and gender-matched subjects/group) are assessed using LC-MS/MS. Absorption is calculated using area under the time-concentration curves (AUC) from samples collected at 0, 3, and 6 h post-ingestion. MetS subjects have ≈6.4-fold higher median vitamin A AUC (retinyl palmitate) versus healthy controls (P = 0.07). Vitamin D2 AUC is unaffected by MetS status (P = 0.48). Untargeted LC-MS lipidomics reveals six phospholipids and one cholesterol ester with concentrations correlating (r = 0.53-0.68; P < 0.001) with vitamin A concentration.
Conclusions:
The vitamin A-phospholipid association suggests increased hydrolysis by PLB, PLRP2, and/or PLA2 IB may be involved in the trend in higher vitamin A bioavailability in MetS subjects. Previously observed differences in circulating levels of these vitamins are likely not due to absorption. Alternate strategies should be investigated to improve FSV status in MetS.
jomamma007
Member
I never want to discredit somebody’s progress, but eliminating 95% of foods would help nearly every American.
Paul Saladino “cured” his eczema with a carnivore diet although he still can’t “tolerate” dairy. Vitamin A can suppress the thyroid if you’re already hypothyroid. That’s why so many on the carnivore diet are saying how liver is giving them bad reactions, because they’re already hypo.
All the most nutritius foods are loaded with A. Cheese, milk, eggs, liver, grass finished beef etc… in fact I saw some studies showing grass finished beef having quite a large amount of Vitamin A so I’m not sure how a diet of beef and rice is even devoid of A. It’s just a carnivore diet with some added white rice (one of the most tolerable carbs and 100-% glucose) to avoid the low carb problems.
You’re not fixing anything, just avoiding things that cause issues. I can also understand if you haven’t gotten progress trying all the Peat things and find something simple like beef and rice and feel better and stick with it. It’s still healthier than SAD.
Paul Saladino “cured” his eczema with a carnivore diet although he still can’t “tolerate” dairy. Vitamin A can suppress the thyroid if you’re already hypothyroid. That’s why so many on the carnivore diet are saying how liver is giving them bad reactions, because they’re already hypo.
All the most nutritius foods are loaded with A. Cheese, milk, eggs, liver, grass finished beef etc… in fact I saw some studies showing grass finished beef having quite a large amount of Vitamin A so I’m not sure how a diet of beef and rice is even devoid of A. It’s just a carnivore diet with some added white rice (one of the most tolerable carbs and 100-% glucose) to avoid the low carb problems.
You’re not fixing anything, just avoiding things that cause issues. I can also understand if you haven’t gotten progress trying all the Peat things and find something simple like beef and rice and feel better and stick with it. It’s still healthier than SAD.
on a long enough timeline, everyone on this thread, and then this entire forum, will come to this conclusion:
amazon right againIt has gotten to a point where everything is being bashed by someone, so all must be toxic. Living in a toxic world will eventually turn you into a toxin: being alive is unhealthy.
I believe that this is why people seek things like killcium supplements, poison A, venom D, seeyafood, coffeen, millk, pootatoes, leaves, cuckoonut products, yellow-red foods, and onions; it takes courage to put an end to it, it's easier to go after destructive habits instead.
isnt white rice difficult to digest, just easier than glutenI never want to discredit somebody’s progress, but eliminating 95% of foods would help nearly every American.
Paul Saladino “cured” his eczema with a carnivore diet although he still can’t “tolerate” dairy. Vitamin A can suppress the thyroid if you’re already hypothyroid. That’s why so many on the carnivore diet are saying how liver is giving them bad reactions, because they’re already hypo.
All the most nutritius foods are loaded with A. Cheese, milk, eggs, liver, grass finished beef etc… in fact I saw some studies showing grass finished beef having quite a large amount of Vitamin A so I’m not sure how a diet of beef and rice is even devoid of A. It’s just a carnivore diet with some added white rice (one of the most tolerable carbs and 100-% glucose) to avoid the low carb problems.
You’re not fixing anything, just avoiding things that cause issues. I can also understand if you haven’t gotten progress trying all the Peat things and find something simple like beef and rice and feel better and stick with it. It’s still healthier than SAD.
what are yellow-red foods?on a long enough timeline, everyone on this thread, and then this entire forum, will come to this conclusion:
amazon right again
md_a
Member
- Joined
- Aug 31, 2015
- Messages
- 468
SARS-CoV2 pathogenesis and effects of vitamin A on the immune system
SARS-CoV2 infection of the respiratory epithelium
Similarly to SARS-CoV1, the cellular infection mechanism of SARS-CoV2 is mainly mediated by the cell-surface receptor angiotensin-converting enzyme 2 (ACE2)(19). SARS-CoV2 uses ACE2 for receptor-mediated cell entry and serine protease transmembrane serine protease 2 (TMPRSS2) for S protein priming(20). Cell entry of SARS-CoV2 can be partially blocked by the clinically proven serine protease inhibitor camostat mesylate, which is active against serine protease transmembrane serine protease 2 (TMPRSS2)(20). Although very low levels of ACE2 protein have been observed in the normal respiratory system(21), the presence of ACE2 protein levels and viral RNA has been confirmed in ciliated epithelial cells, indicating that low-level protein expression in upper airway epithelial cells facilitates infection of ciliated cells, followed by a rapid interferon-induced increase of ACE2 expression in lower airway ciliated and type-2 alveolar epithelial cells, potentially allowing SARS-CoV2 to spread across the respiratory mucosa(22). The cell-surface ACE2 receptor internalises on binding to the SARS-CoV spike protein, leading to ACE2 receptor down-regulation(23,24) . The SARS-CoV spike protein-mediated ACE2 down-regulation could contribute to the severity of lung pathologies such as pulmonary fibrosis and acute respiratory distress syndrome, since the counterbalance of ACE2 on angiotensin II production in the renin–angiotensin system is deregulated, affecting its ability to modulate the innate immune system and to regulate inflammation(19,25,26) . ACE2 knockout mice infected with SARS-CoV1 show reduced pathological alterations in the lung(19). Increased incidence of intravascular coagulation with SARS-CoV2 infection could also be mediated via ACE2, since ACE2 deficiency is associated with up-regulation of putative mediators of atherogenesis, such as cytokines and adhesion molecules, and reduced ACE expression is observed in established atherosclerotic plaques(27). Finally, the observed difference in SARS-CoV2 severity between children and adults could in part be attributed to the lower expression of the SARS-CoV2-targeted receptor ACE2 and serine protease serine protease transmembrane serine protease 2 (TMPRSS2) in nasal and bronchial airways in children compared with adults(28).Vitamin A and epithelial barriers
VA is needed to maintain healthy respiratory and intestinal epithelial barriers(17). VA deficiency may not produce obvious changes in healthy epithelial surfaces, but following viral infection in the intestine, for example, pathological changes to infected epithelial surfaces were found to be much greater in VA-deficient than in control mice(29). In the respiratory tract, VA deficiency also increases epithelial damage and impairs recovery, sometimes leading to squamous metaplasia in alveoli and airways, following noxious exposures such as ozone treatment(30) and infection with influenza virus(31). Sputum (mucus) also provides a solid physical barrier to pathogens(32) and contains many of the macromolecules of the innate immune system(33). Sputum is a mixture of oligomeric mucins, MUC2, MUC5AC and MUC5B which are synthesised and secreted by the differentiated mucociliary epithelium(34). Regulation of mucin production is induced by all-trans retinoic acid via retinoic acid receptor alpha as the major retinoid receptor subtype(35). Importantly, moderate VA supplementation, but not high-dose VA supplementation or VA deficiency, improves mucin secretion by regulating the gene expression of cytokines and epithelial growth factors(36).The VA metabolite all-trans retinoic acid has also been shown to up-regulate the expression of ACE2(37,38) , resulting in the reduction of blood pressure and the attenuation of myocardial damage in spontaneously hypertensive rats(37). Increased levels of ACE2 after VA supplementation could have two effects: 1) it could increase the risk of SARS-CoV2 infection at the time of virus exposure(39), particularly in individuals who have an adequate VA status; or 2) it could reduce the risk of sympathetic over-activation seen in severe SARS-CoV2 infection, obese and diabetic patients(40–42) through VA supplementation that activates the ACE2-Ang 1–7-Mas axis.
VA is also important during embryonic lung development(43); thus, the role in tissue repair in postnatal life is not surprising. A recent review highlights the potential role of VA and retinoic acid as possible therapeutic interventions to prevent pulmonary fibrosis following lung injury(44), suggesting a role for VA in recovery from severe COVID-19 disease. Lipofibroblasts are the retinoid storage cells of the lung, similar to stellate cells of the liver(18), and contain many components of the retinoid signalling pathway including receptors and binding proteins(45). Importantly, the defining characteristic of hepatic fibrosis is the loss of VA from stellate cells(46). Similarly, chronic nutritional VA deficiency results in pulmonary fibrosis through decreased alveolar septation, squamous metaplasia of the respiratory epithelium and a thickening of the alveolar basement membrane followed by an increase in collagens and a deposition of ectopic collagen fibrils(14). Pulmonary fibrosis is characterised by conversion of lipofibroblasts to FGF10-expressing myofibroblasts localised to αSMA expressing cells, down-regulation of PPARγ and activation of TGF-β signalling(47). PPARγ activation reverts fibrosis in both the lung and the liver through inhibition of αSMA, type I collagen and TGF-β expression(46,48) . The beneficial effects of retinoids on pulmonary fibrosis may be through activation of the retinoid X receptor via 9-cis-retinoic acid(49).
Innate immune response to SARS-CoV2 infection
The immune response to respiratory viral infections, including coronaviruses, is initiated by barrier defences at the site of initial virus replication(50). SARS-CoV2 infects ciliated epithelial cells in the upper respiratory tract and alveolar type II pneumocytes in the lower respiratory tract via its cellular receptor ACE2(51). SARS-CoV2 thus first engages the innate immune system in respiratory epithelial cells and adjacent macrophages. Viral RNA in the cytoplasm or endosomes is recognised by pattern-recognition receptors including RIG-1, TLR3, TLR7 and TLR9. These receptors trigger transcription of type I and type III interferon (IFN) which act via cellular receptors to induce anti-viral programmes in infected and adjacent cells that render these cells refractory to viral infection. However, coronaviruses, including SARS-CoV2, can act on these pathways to block induction of anti-viral activity, which may be a factor in the development of more severe diseases. In addition to type I and III IFN, pattern-recognition receptor activation triggers production of other cytokines, including TNF-α, IL-1, IL-6 and IL-18 by innate immune cells, particularly macrophages. These cytokines help initiate inflammation, including recruitment of other immune cells to the site of initial infection. Innate lymphoid cells found at tissue sites are often involved in the initial response to viral (and other) infections, but their role in SARS-CoV2 infection is uncertain(52,53) .Effects of vitamin A on innate immunity
Many cells of the innate immune system, including innate lymphoid cells, macrophages and granulocytes, are affected by VA. Type 1 innate lymphoid cells, including natural killer (NK) cells, play a role in responses to viral infection and their activity and number in peripheral blood is decreased by VA deficiency(54), while retinoic acid treatment can also dampen activity(55). Neutrophil and macrophage function is also directly affected by VA. Phagocytic and bacterial-killing activity of these cells is impaired by VA deficiency, leading to decreased resistance to some infections, though production of the CD4+ T-helper type 1 (Th1)-promoting cytokine IL-12 can be increased, causing increased type 1 inflammation(17). Ex vivo and animal studies show that treatment of macrophages with retinoic acid can have anti-inflammatory effects, dampening production of IL-12 and pro-inflammatory cytokines such as TNF-α, as well as increasing production of the regulatory cytokine IL-10(55). Other effects on macrophage phenotype are also caused by retinoic acid. For example, in Leishmania infection of mice, retinoic acid treatment diminishes the development of M1 macrophages, a component of protective type 1 immunity in this model system, and increases the development of M2 macrophages and type 2 immunity, which is not protective. Thus, retinoic acid intervention in this system decreases resistance to infection(56). Conversely, in Schistosoma mansoni infection of mice, VA deficiency exacerbates disease due to excessive inflammation and tissue damage, while retinoic acid treatment increases conversion of macrophages into a tissue-resident phenotype that dampens inflammation and allows control of infection and enhancement of tissue repair(57). While many of the effects of retinoic acid on innate immune cells can be seen as anti-inflammatory, a lesson from these and other studies is that intervention with retinoic acid during infections can have unpredictable effects on the course of a particular infection depending on the specifics of the immune response that are responsible for pathogen clearance. This topic has been discussed in a recent review(58).Adaptive immune response to SARS-CoV2 infection
Dendritic cells link the initial anti-viral response of the innate immune system to development of adaptive immunity, involving B and T lymphocytes, that will help clear infections that cannot be resolved by innate immunity alone. Adaptive immunity also has a memory component that, in the case of most acute viral infections, provides a rapid and robust response upon subsequent exposure to the same virus, typically providing protection against symptomatic infection. Such responses develop for other coronaviruses(50), and hopefully, this will also be true of SARS-CoV2.To initiate adaptive immunity, dendritic cells at the site of infection are also activated by pattern-recognition receptor-mediated recognition of viruses, as well as by local cytokine production, and deliver viral antigen from the site of infection to a regional lymph node to initiate development of virus-specific effector and memory T cells, as well as memory B cells that results in development of antibody-producing plasma cells. Immunologists recognise three basic types of protective immunity against infectious diseases, types 1, 2 and 3(59). Type 1 immunity is typically induced in response to viruses and other intracellular pathogens and involves development of Th1 cells producing the signature cytokine IFN-γ and CD8+ cytotoxic T-lymphocyte which can directly kill virus-infected cells, and a robust serum IgG antibody response, often targeting the epitope of the viral glycoprotein involved in binding to its cellular receptor (the spike
Research on the adaptive immune response to SARS-CoV2 is in an early state, but two recent reviews indicate that patients recovering from COVID-19 have the Th1, CD8+ cytotoxic T-lymphocyte and neutralising antibody responses expected of a typical type 1 response against a viral infection(52,60) . Briefly, Th1 responses predominate over Th2 and Th17, as expected, and viral epitopes from all major structural proteins, including spike (S), matrix (M) and nucleocapsid (N), are seen in recovering patients. CD8 T-cell responses are also seen against the same proteins. The magnitude of the response is somewhat greater in patients with more severe disease, which could be due to greater antigenic stimulation. Similar patterns of response were seen against SARS-CoV1 in recovered SARS patients(61), and protection against death by CD8 cells was demonstrated in a mouse model(62). Similar CD4 and CD8 responses were seen against MERS-CoV in MERS survivors 0·5–2 years after infection(63). These findings suggest that patients recovering from SARS-CoV2 infection may have protective T-cell memory responses. In contrast to the potential benefit of such Th1 responses, some evidence from SARS patients(64) as well as preliminary work from COVID-19 patients cited in a recent review(60) suggests that Th2 responses may be associated with poor outcomes to infection. B-cell responses develop in response to SARS-CoV2, as demonstrated by the serum IgM and IgG antibody responses that are seen within 2 weeks of infection, as is also seen with MERS-CoV and SARS-CoV1(65). Antibodies are directed primarily against the S and N proteins, and the ACE2-binding site is quite immunogenic, resulting in robust neutralising antibody responses(52).
Effects of vitamin A on adaptive immunity
As discussed above, dendritic cells are a key bridge from innate to adaptive immunity during infection and immunisation. Dendritic cells provide the signals necessary to stimulate development of pathogen-appropriate adaptive immune responses. These signals include peptide antigen to provide pathogen-specificity (i.e. recognition of a specific strain of the flu virus by memory T cells), co-stimulation to insure continued proliferation of developing T cells and specific cytokines to steer CD4 T-cell development towards the pathogen-appropriate Th1, Th2, Th17, Treg and CD4+ follicular-helper T phenotypes. In addition, a fourth signal that is often provided by dendritic cells, particularly in the intestinal and other mucosal-associated immune tissues, is retinoic acid(66), the production of which is diminished during VA deficiency. Retinoic acid affects several aspects of T-cell development, including modification of T-cell phenotype development and mucosal homing of lymphocytes. These effects have recently been reviewed(12). They are pleiotropic and depend on modifying factors (e.g. the local cytokine environment), but several main points are clear.Perhaps, the most consistent and biologically important effect of retinoic acid produced by dendritic cells, and a principal reason for retinoic acid production being constitutive in mucosal dendritic cells, is that retinoic acid induces transcription of two mucosal-homing molecules on lymphocytes, chemokine receptor 9 and α4β7 integrin. Thus, memory T cells, B cells and antibody-producing plasma cells which develop in the intestine or respiratory tract (both are initiating sites of the common mucosal immune response) will preferentially recirculate to these sites, whereas T cells developing from systemic lymph nodes are less likely to do so(66). Mucosal infections often trigger secretory IgA responses, and the plasma cells responsible for secreting IgA are found at submucosal sites due to the imprinting of chemokine receptor 9 and α4β7 integrin expression. This mechanism may explain why VA deficiency sharply reduces the secretory IgA response to influenza A virus infection in the nasal mucosa of mice(67). Furthermore, impaired IgA antibody and infrequent virus-specific CD8+ T response following respiratory virus infection induced a cytokine storm in the upper respiratory tract of VA-deficient mice(68). Retinoic acid also enhances intestinal homing of other cell types, including Treg cells(69).
Second, VA deficiency has contrasting effects on Th1/Th2 development, with deficiency not affecting or sometimes enhancing Th1 development (via increased IL-12 production, as discussed above) but dampening Th2 development, particularly during the early stages of phenotype commitment immediately after antigenic stimulation. In contrast, retinoic acid, or high-level dietary VA, can enhance type 2 immunity(70). However, retinoic acid, at a somewhat later stage in development of Th1 memory cells, can help stabilise phenotype commitment to the Th1 lineage(71). Similarly, effects of VA on CD8+ cytotoxic T-lymphocyte development, a key component of type 1, anti-viral immunity are mixed(12). Using model viral infections to explore the effects of VA deficiency has shown no impairment of clearance of acute influenza A virus infection in mice, a slight enhancement of some aspects of the type 1 response squamous metaplasia during recovery(31). Using chronic lymphocytic choriomeningitis virus infection in mice also showed an elevated type 1 response, more severe pathological changes, higher virus load and increased mortality, effects which were reversed with retinoic acid(72).
Third, VA regulates the balance between Th17 and Treg development. Both cell types often develop in the intestinal lymphoid tissue (as well as at other sites) with Treg cells being predominant when inflammation is minimal and constitutive TGF-β and retinoic acid production bias memory T-cell development towards a Treg phenotype which is appropriated for antigens derived from food and commensal bacteria. However, when inflammation develops, IL-6 is produced and this shifts the balance towards Th17. During VA deficiency, Treg development is diminished because retinoic acid production is reduced(55).
Fourth, retinoic acid affects B cell development, and VA deficiency decreases the antibody response to T-cell-dependent antigens, in particular(12). While VA deficiency in mice slightly enhances type 1-related IgG2a antibody responses, the IgG1 response is diminished and, in particular, the IgA response is specifically diminished because retinoic acid can enhance class-switching to IgA(73,74) .
Contribution of the immune system to COVID-19 pathogenesis
Approximately 5 % of COVID-19 patients, many with underlying conditions such as CVD, diabetes mellitus and obesity, develop severe disease with a high risk of death. Male sex and age above 65 years are also risk factors(6). The pathogenesis of severe disease involves excessive activation of the immune system that can result in immunopathology that increases disease severity, as has been reviewed recently(52,75–77) . During the initial innate immune response, a robust IFN type I/III response appears to predict a milder course of disease, presumably by controlling initial virus replication and allowing development of the adaptive response. However, patients with severe disease are more likely to have a lower initial IFN type I/II response (though it may be elevated later) which may result in a failure to control initial viral replication. Prolonged viral replication then appears to result in high-level production of pro-inflammatory cytokines (including TNF-α, IL-1, IL-6 and many others), likely produced by activated macrophages but perhaps by other innate cells such as neutrophils that may be attracted to the lungs. This local hyper-inflammation may dampen development of adaptive immunity and in patients with severe disease lymphopenia, including low levels of T cells, is pronounced. During this ‘cytokine storm’ with activation of innate immune cells, a system response develops that includes elevation of acute phase proteins, particularly CRP, and activation of the complement system which may result in vascular leakage as well as diffuse intravascular coagulation which can cause organ damage thus increasing disease severity. Developing therapeutic interventions to dampen this immune-mediated pathology is a high priority of current research. Potential drug targets include the NF-κB pathway to attenuate TNF-α and IL-6 expression, the JAK/STAT signalling pathway and the sphingosine-1-phosphate receptor 1 pathway(78). The JAK/STAT and sphingosine-1-phosphate receptor 1 signalling pathways are of particular interest since SARS-CoV2 potential down-regulation of ACE2 expression could result in over-production of angiotensin II via the related ACE enzyme, leading to enhanced IL-6 production. JAK/STAT inhibitors such as Baricitinib or sphingosine-1-phosphate receptor 1 receptor agonists such as Fingolimod could attenuate the cytokine storm(78).Possible implications of vitamin A deficiency for immune response to SARS-CoV2
The effects of VA in the immune system discussed above have several possible implications for individuals with VA deficiency infected by SARS-CoV2. First, VA deficiency is not likely to impair a type 1 anti-viral response to SARS-CoV2, but could increase the severity of type 1 inflammation and tissue damage in the lungs following viral infection. Second, after clearance of SARS-CoV2 infection, VA deficiency could impair repair of damaged alveolar pneumocytes and airway epithelium. Third, protective immunity to SARS-CoV2 could be impaired by VA deficiency, particularly the mucosal IgA response, which could be important in resistance to reinfection, though development of memory Th1 and CD8+ cytotoxic T-lymphocyte response might also be affected.Effect of infections on vitamin A metabolism and status
VA is absorbed in the intestine with high efficiency in healthy individuals (>80 %) and mainly stored in the liver (>90 %), with smaller amounts stored in intestine, kidney, adipose tissue and the lung(79). Release of serum VA bound to retinol-binding protein (RBP4) is homoeostatically controlled and does not change over a wide range of liver reserves(79). During infection, the acute protein response increases the synthesis of hepatic inflammatory cytokines, but at the same time reduces the release of negative acute phase proteins such as RBP4. The subsequent reduction of holo-RBP4 (retinol bound to apo-RBP4) causes a decline in circulating VA even before the acute-phase proteins CRP and AGP have reached peak concentrations(80). Reduced serum retinol and RBP4 concentrations could potentially overestimate the prevalence of VA deficiency in populations with high levels of inflammation(81). Severe infections have also shown to reduce food intake(80), impair absorption by 20–30 %(15), increase metabolic requirements or catabolic losses(80) and result in substantial urinary losses(17,82) . Importantly, respiratory infections combined with low dietary VA intake could deplete liver VA stores in COVID-19 patients suffering from pneumonia to levels associated with VA deficiency, since urinary losses of >1000 μg retinol/d have been observed in 24 % of ICU patients with pneumonia and sepsis, representing a higher amount than the RDA for VA(82). We estimate that this level of urinary loss, combined with a postulated decrease in intake of approximately 50 % of the RDA for women hospitalised with COVID-19, could lead to a loss of approximately 1350 μg/d from liver stores. Such losses would lead to deficient liver stores (<20 μg/g) in approximately 3 weeks for a 61 kg women with relatively low pre-existing liver stores (40 μg/g; estimated liver weight of 2·4 % of body weight). Using data on estimated liver stores among US women of 129 (sd 89) μg/g who consumed high levels of VA (a mean intake of 173 (sd 111) % of the RDA of 700 μg retinol)(83), we estimate that approximately 16 % of women (those at least one sd below the mean) in a population with similarly high intake would have reserves of 40 μg/g or less. In populations consuming lower levels of VA, the percentage of individuals at risk for systemic VA deficiency would be higher. It is important to note however that these systemic effects of VA are different to tissue-specific VA deficiency that could develop following COVID-19 infection (Fig. 1). First, pulmonary fibrosis induced by SARS-CoV2 is characterised by the conversion of lipofibroblasts to activated myofibroblasts that excessively deposit extracellular matrix proteins and lose their retinoid stores(45,48) . Second, inflammation induced reduction of retinoic acid uptake and metabolism to polar metabolites(80) could affect the ability of the lung to regenerate from pulmonary fibrosis, since lipofibroblasts rely on retinoids to initiate, coordinate and regulate alveolar septal eruption and alveolo-genesis(45). Third, the ability to deliver retinoids to the lung is compromised during severe infection due to the decline in circulating VA(80). Nutritionists have long recognised that acute infection exacerbates the risk of malnutrition, as there is potential to fall into a ‘vicious circle’ of acute illness, deficiency and susceptibility to secondary infection. However, the timing of VA supplementation during SARS-CoV2 infection and the recovery period may be critical, as early intervention during the infectious period may be contraindicative due to its impact on the ACE2 receptor(37,38) , but intervention during the recovery period may be critical to address the potentially developing tissue-specific VA deficiency and its effects on the respiratory immune response. Importantly, VA supplementation may not be beneficial depending on age and co-morbidity, since VA supplementation can increase the risk of acute respiratory tract infections in children with adequate VA status(84) and can delay recovery from community-acquired pneumonia in children(85) and respiratory syncytial virus infection in infants(86). However, VA supplementation was associated with improved recovery from Ebola virus infection in adults(87) and can have beneficial effects in obesity by correcting tissue VA deficiency, altering lung immune cell composition, improving vaccine responses and assisting in respiratory virus clearance(88). In particular, high-dose VA supplementation during recovery from measles has been shown to decrease mortality and improve recovery, including decreasing the severity of secondary bacterial pneumonia(89). Delivering VA through inhalation may be more efficient to address the immunological lesions in VA-deficient individuals(90,91) particularly since inflammation also reduces retinoic acid uptake and metabolism to polar metabolites(80).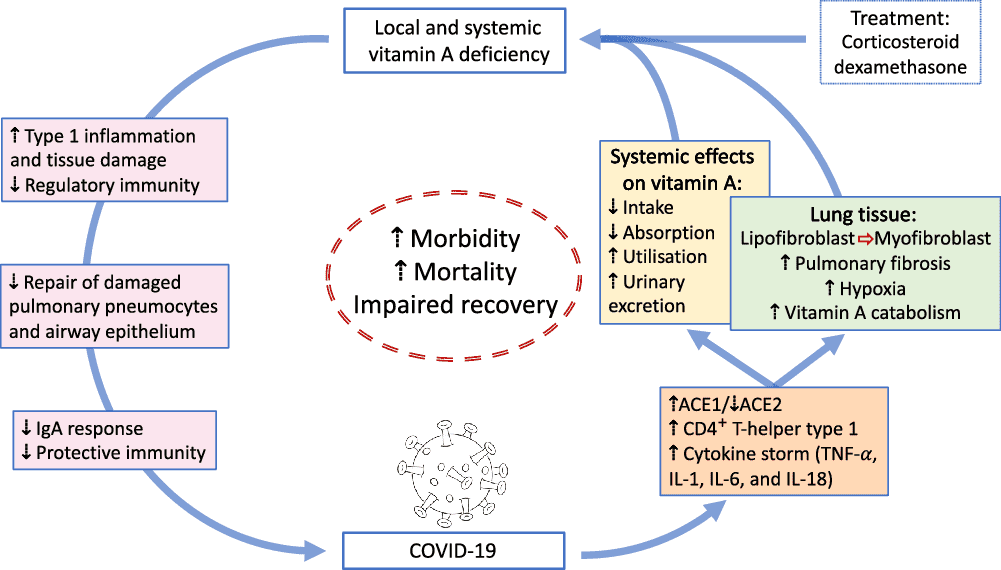
Fig. 1. Overview of potential interactions between vitamin A and COVID-19. SARS-CoV2 uses angiotensin-converting enzyme 2 (ACE2) for receptor-mediated cell entry, leading to ACE2 down-regulation and a deregulation of the renin–angiotensin system. Viral RNA triggers the production of CD4+ T-helper type 1 cells and cytokines, including TNF-α, IL-1, IL-6 and IL-18. In the lung, SARS-CoV2 can lead to severe acute respiratory syndrome due to extensive pulmonary fibrosis promoted by enhanced lipofibroblast–myofibroblast transition. Since lipofibroblasts rely on retinoids to initiate, coordinate and regulate alveolar septal eruption and alveolo-genesis, the loss of retinoids during the virus-induced lipofibroblast–myofibroblast transition could impair the ability of the lung to repair damaged epithelial surfaces, potentially leading to long-lasting scarring, lung fibrosis and reduced pulmonary capacity, which could manifest into a ‘long COVID’ effect. Treatment of COVID-19 patients with dexamethasone could further increase localised vitamin A deficiency through the reduction of retinoid-binding proteins and receptors. On the other hand, SARS-CoV2 could also lead to systemic vitamin A deficiency through a combination of increased urinary losses, reduced intake and absorption and increased utilisation. The effects of local and systemic vitamin A deficiency have been shown to reduce the ability of the immune system to maintain healthy respiratory and intestinal epithelial barriers, activity and numbers of type 1 innate lymphoid cells and secretory IgA responses to virus infection. This vicious cycle of increased vitamin A deficiency and decreased regulatory and protective immunity impairs recovery and is likely to increase morbidity and mortality.
Interaction of vitamin A with olfactory function
SARS-CoV2 causes olfactory and/or gustatory dysfunctions in COVID-19 patients(92–95). A recent meta-analysis of eight studies confirmed that patients with COVID-19 had significantly higher risks of developing olfactory and/or gustatory dysfunctions compared with normal subjects (OR of 65·9) or patients with acute respiratory infection without detectable virus (OR of 11·3)(94). On average, 50 % of COVID-19 patients suffered from olfactory and/or gustatory dysfunctions(94), although olfactory impairment was reported to vary between 4·8 % in China, 68 % in the USA and 85·6 % in Europe(93). The mean time from olfactory loss to recovery onset was reported as 12 d, with complete olfactory recovery in only 51 % of patients(93). Viral infections are a common cause of olfactory dysfunction(96), although the pathogenesis of sensory loss after viral infections is not well characterised(92,95) . It is assumed that the nasal epithelium and olfactory bulb could be critical in the pathological development of COVID-19, with mild outcomes of COVID-19 leading to localised effects with mild effects on olfactory function. Interestingly, the effects of SARS-CoV2 on chronic olfactory impairment increase with age, affecting up to 50 % of people aged ≥65 years and >80 % of people aged ≥80 years(92).VA supplementation may play an important role in the regeneration of olfactory receptor neurons, particularly since retinoic acid signalling is involved in neuronal regeneration(97). Indeed, a recent retrospective cohort analysis in patients with post-infectious olfactory dysfunction indicated that topically applied VA at a dose of 10 000 IU/d for 8 weeks improved clinical outcome(98). This beneficial effect of VA to improve olfactory dysfunction is supposedly due to increased local concentration of VA at the olfactory neuroepithelium(98). Thus, similarly for treating VA deficiency in the lung, topical administration of VA may increase bioavailability and reduce toxic side effects of high-dose systemic VA therapy needed to overcome localised VA deficiency(91,98,99) . However, since no studies have investigated the benefit of topical application of VA in COVID-19 patients suffering from olfactory dysfunction, prospective, double-blind and placebo-controlled trials are needed to confirm if VA therapy would be of benefit to those patients that struggle to recover from olfactory impairment.
Interaction of vitamin A with current SARS-CoV2 medication
The RECOVERY trial results demonstrated that low-dose dexamethasone reduced the death rate of patients that require respiratory support(3). However, although corticosteroids have anti-inflammatory, antioxidant, pulmonary vasodilator and anti-edematous effects(100), their impact on limiting immune responses might counteract viral clearance(101). Previous studies associate the use of corticosteroid in SARS, MERS and influenza with a higher risk of a delayed viral clearance and long-term complications in survivors(102). Furthermore, dexamethasone decreases alveolar numbers, and this effect on alveolar architecture can be long term or permanent(18). The drug also reduces retinoid-binding proteins and receptors in mice(18), which potentially could affect the metabolism of VA in affected tissues. Since chronic nutritional VA deficiency can result in pulmonary fibrosis(14), long-term complications in survivors after dexamethasone treatment could include lung tissue scaring due to medication-induced VA deficiency. On the other hand, supplementation with a nutrient-metabolite combination containing VA and retinoic acid in a 10:1 molar ratio (VARA) was effective in increasing the concentration of VA to a level that was not antagonised by dexamethasone, probably since the VARA-induced lung tissue VA accumulation compensated the VA loss caused by dexamethasone(103). Importantly, the delivery of retinoids to the lung tissue determines whether or not the negative side effects of dexamethasone on lung VA levels can be overcome. Treatment with retinoic acid was shown to reverse pulmonary emphysema(104), but caused significant toxic side effects(105). To obtain biologically effective levels in the lung, inhalation of retinoids rather than oral administration may be a better approach(91), particularly since this approach induces cellular retinol-binding protein 1 protein expression which was shown to be reduced following dexamethasone treatment(18). However, effectiveness of either VARA or retinoic acid inhalation to optimise lung VA stores would need to be tested in COVID-19-affected individuals who have been treated with dexamethasone.Vitamin A in resistance to and recovery from infection: relevance to SARS-CoV2 | British Journal of Nutrition | Cambridge Core
Vitamin A in resistance to and recovery from infection: relevance to SARS-CoV2
mate whyd you cross everything out with the lines through itSARS-CoV2 pathogenesis and effects of vitamin A on the immune system
SARS-CoV2 infection of the respiratory epithelium
Similarly to SARS-CoV1, the cellular infection mechanism of SARS-CoV2 is mainly mediated by the cell-surface receptor angiotensin-converting enzyme 2 (ACE2)(19). SARS-CoV2 uses ACE2 for receptor-mediated cell entry and serine protease transmembrane serine protease 2 (TMPRSS2) for S protein priming(20). Cell entry of SARS-CoV2 can be partially blocked by the clinically proven serine protease inhibitor camostat mesylate, which is active against serine protease transmembrane serine protease 2 (TMPRSS2)(20). Although very low levels of ACE2 protein have been observed in the normal respiratory system(21), the presence of ACE2 protein levels and viral RNA has been confirmed in ciliated epithelial cells, indicating that low-level protein expression in upper airway epithelial cells facilitates infection of ciliated cells, followed by a rapid interferon-induced increase of ACE2 expression in lower airway ciliated and type-2 alveolar epithelial cells, potentially allowing SARS-CoV2 to spread across the respiratory mucosa(22). The cell-surface ACE2 receptor internalises on binding to the SARS-CoV spike protein, leading to ACE2 receptor down-regulation(23,24) . The SARS-CoV spike protein-mediated ACE2 down-regulation could contribute to the severity of lung pathologies such as pulmonary fibrosis and acute respiratory distress syndrome, since the counterbalance of ACE2 on angiotensin II production in the renin–angiotensin system is deregulated, affecting its ability to modulate the innate immune system and to regulate inflammation(19,25,26) . ACE2 knockout mice infected with SARS-CoV1 show reduced pathological alterations in the lung(19). Increased incidence of intravascular coagulation with SARS-CoV2 infection could also be mediated via ACE2, since ACE2 deficiency is associated with up-regulation of putative mediators of atherogenesis, such as cytokines and adhesion molecules, and reduced ACE expression is observed in established atherosclerotic plaques(27). Finally, the observed difference in SARS-CoV2 severity between children and adults could in part be attributed to the lower expression of the SARS-CoV2-targeted receptor ACE2 and serine protease serine protease transmembrane serine protease 2 (TMPRSS2) in nasal and bronchial airways in children compared with adults(28).
Vitamin A and epithelial barriers
VA is needed to maintain healthy respiratory and intestinal epithelial barriers(17). VA deficiency may not produce obvious changes in healthy epithelial surfaces, but following viral infection in the intestine, for example, pathological changes to infected epithelial surfaces were found to be much greater in VA-deficient than in control mice(29). In the respiratory tract, VA deficiency also increases epithelial damage and impairs recovery, sometimes leading to squamous metaplasia in alveoli and airways, following noxious exposures such as ozone treatment(30) and infection with influenza virus(31). Sputum (mucus) also provides a solid physical barrier to pathogens(32) and contains many of the macromolecules of the innate immune system(33). Sputum is a mixture of oligomeric mucins, MUC2, MUC5AC and MUC5B which are synthesised and secreted by the differentiated mucociliary epithelium(34). Regulation of mucin production is induced by all-trans retinoic acid via retinoic acid receptor alpha as the major retinoid receptor subtype(35). Importantly, moderate VA supplementation, but not high-dose VA supplementation or VA deficiency, improves mucin secretion by regulating the gene expression of cytokines and epithelial growth factors(36).
The VA metabolite all-trans retinoic acid has also been shown to up-regulate the expression of ACE2(37,38) , resulting in the reduction of blood pressure and the attenuation of myocardial damage in spontaneously hypertensive rats(37). Increased levels of ACE2 after VA supplementation could have two effects: 1) it could increase the risk of SARS-CoV2 infection at the time of virus exposure(39), particularly in individuals who have an adequate VA status; or 2) it could reduce the risk of sympathetic over-activation seen in severe SARS-CoV2 infection, obese and diabetic patients(40–42) through VA supplementation that activates the ACE2-Ang 1–7-Mas axis.
VA is also important during embryonic lung development(43); thus, the role in tissue repair in postnatal life is not surprising. A recent review highlights the potential role of VA and retinoic acid as possible therapeutic interventions to prevent pulmonary fibrosis following lung injury(44), suggesting a role for VA in recovery from severe COVID-19 disease. Lipofibroblasts are the retinoid storage cells of the lung, similar to stellate cells of the liver(18), and contain many components of the retinoid signalling pathway including receptors and binding proteins(45). Importantly, the defining characteristic of hepatic fibrosis is the loss of VA from stellate cells(46). Similarly, chronic nutritional VA deficiency results in pulmonary fibrosis through decreased alveolar septation, squamous metaplasia of the respiratory epithelium and a thickening of the alveolar basement membrane followed by an increase in collagens and a deposition of ectopic collagen fibrils(14). Pulmonary fibrosis is characterised by conversion of lipofibroblasts to FGF10-expressing myofibroblasts localised to αSMA expressing cells, down-regulation of PPARγ and activation of TGF-β signalling(47). PPARγ activation reverts fibrosis in both the lung and the liver through inhibition of αSMA, type I collagen and TGF-β expression(46,48) . The beneficial effects of retinoids on pulmonary fibrosis may be through activation of the retinoid X receptor via 9-cis-retinoic acid(49).
Innate immune response to SARS-CoV2 infection
The immune response to respiratory viral infections, including coronaviruses, is initiated by barrier defences at the site of initial virus replication(50). SARS-CoV2 infects ciliated epithelial cells in the upper respiratory tract and alveolar type II pneumocytes in the lower respiratory tract via its cellular receptor ACE2(51). SARS-CoV2 thus first engages the innate immune system in respiratory epithelial cells and adjacent macrophages. Viral RNA in the cytoplasm or endosomes is recognised by pattern-recognition receptors including RIG-1, TLR3, TLR7 and TLR9. These receptors trigger transcription of type I and type III interferon (IFN) which act via cellular receptors to induce anti-viral programmes in infected and adjacent cells that render these cells refractory to viral infection. However, coronaviruses, including SARS-CoV2, can act on these pathways to block induction of anti-viral activity, which may be a factor in the development of more severe diseases. In addition to type I and III IFN, pattern-recognition receptor activation triggers production of other cytokines, including TNF-α, IL-1, IL-6 and IL-18 by innate immune cells, particularly macrophages. These cytokines help initiate inflammation, including recruitment of other immune cells to the site of initial infection. Innate lymphoid cells found at tissue sites are often involved in the initial response to viral (and other) infections, but their role in SARS-CoV2 infection is uncertain(52,53) .
Effects of vitamin A on innate immunity
Many cells of the innate immune system, including innate lymphoid cells, macrophages and granulocytes, are affected by VA. Type 1 innate lymphoid cells, including natural killer (NK) cells, play a role in responses to viral infection and their activity and number in peripheral blood is decreased by VA deficiency(54), while retinoic acid treatment can also dampen activity(55). Neutrophil and macrophage function is also directly affected by VA. Phagocytic and bacterial-killing activity of these cells is impaired by VA deficiency, leading to decreased resistance to some infections, though production of the CD4+ T-helper type 1 (Th1)-promoting cytokine IL-12 can be increased, causing increased type 1 inflammation(17). Ex vivo and animal studies show that treatment of macrophages with retinoic acid can have anti-inflammatory effects, dampening production of IL-12 and pro-inflammatory cytokines such as TNF-α, as well as increasing production of the regulatory cytokine IL-10(55). Other effects on macrophage phenotype are also caused by retinoic acid. For example, in Leishmania infection of mice, retinoic acid treatment diminishes the development of M1 macrophages, a component of protective type 1 immunity in this model system, and increases the development of M2 macrophages and type 2 immunity, which is not protective. Thus, retinoic acid intervention in this system decreases resistance to infection(56). Conversely, in Schistosoma mansoni infection of mice, VA deficiency exacerbates disease due to excessive inflammation and tissue damage, while retinoic acid treatment increases conversion of macrophages into a tissue-resident phenotype that dampens inflammation and allows control of infection and enhancement of tissue repair(57). While many of the effects of retinoic acid on innate immune cells can be seen as anti-inflammatory, a lesson from these and other studies is that intervention with retinoic acid during infections can have unpredictable effects on the course of a particular infection depending on the specifics of the immune response that are responsible for pathogen clearance. This topic has been discussed in a recent review(58).
Adaptive immune response to SARS-CoV2 infection
Dendritic cells link the initial anti-viral response of the innate immune system to development of adaptive immunity, involving B and T lymphocytes, that will help clear infections that cannot be resolved by innate immunity alone. Adaptive immunity also has a memory component that, in the case of most acute viral infections, provides a rapid and robust response upon subsequent exposure to the same virus, typically providing protection against symptomatic infection. Such responses develop for other coronaviruses(50), and hopefully, this will also be true of SARS-CoV2.
To initiate adaptive immunity, dendritic cells at the site of infection are also activated by pattern-recognition receptor-mediated recognition of viruses, as well as by local cytokine production, and deliver viral antigen from the site of infection to a regional lymph node to initiate development of virus-specific effector and memory T cells, as well as memory B cells that results in development of antibody-producing plasma cells. Immunologists recognise three basic types of protective immunity against infectious diseases, types 1, 2 and 3(59). Type 1 immunity is typically induced in response to viruses and other intracellular pathogens and involves development of Th1 cells producing the signature cytokine IFN-γ and CD8+ cytotoxic T-lymphocyte which can directly kill virus-infected cells, and a robust serum IgG antibody response, often targeting the epitope of the viral glycoprotein involved in binding to its cellular receptor (the spikeprotein in the case of SARS-CoV2), thus neutralising the ability of the virus to infect cells. Macrophages activated by Th1 cells at sites of infection are also a key component of type 1 immunity. Type 2 immunity provides protection against intestinal parasites, involves development of CD4+ Th2 cells producing IL-4, -5 and -13, induction of an antibody response involving IgE and activation of eosinophils and basophils at mucosal surfaces, near the sites of pathogen exposure. Type 3 immunity targets extracellular pathogens, involves development of CD4+ Th17 cells producing IL-17 and IL-22, development of appropriate antibody responses and engagement of mucosal epithelial cells and neutrophils to provide barrier protection. CD4+ follicular-helper T cells develop during all of these responses to provide help for development of antigen-specific memory B cells and a robust antibody response. CD4+ regulatory (Treg) cells also develop during most adaptive responses to provide a pathogen-specific cell type capable of dampening pro-inflammatory immunity by direct cell–cell interaction or production of the cytokines IL-10 and TGF-β.
Research on the adaptive immune response to SARS-CoV2 is in an early state, but two recent reviews indicate that patients recovering from COVID-19 have the Th1, CD8+ cytotoxic T-lymphocyte and neutralising antibody responses expected of a typical type 1 response against a viral infection(52,60) . Briefly, Th1 responses predominate over Th2 and Th17, as expected, and viral epitopes from all major structural proteins, including spike (S), matrix (M) and nucleocapsid (N), are seen in recovering patients. CD8 T-cell responses are also seen against the same proteins. The magnitude of the response is somewhat greater in patients with more severe disease, which could be due to greater antigenic stimulation. Similar patterns of response were seen against SARS-CoV1 in recovered SARS patients(61), and protection against death by CD8 cells was demonstrated in a mouse model(62). Similar CD4 and CD8 responses were seen against MERS-CoV in MERS survivors 0·5–2 years after infection(63). These findings suggest that patients recovering from SARS-CoV2 infection may have protective T-cell memory responses. In contrast to the potential benefit of such Th1 responses, some evidence from SARS patients(64) as well as preliminary work from COVID-19 patients cited in a recent review(60) suggests that Th2 responses may be associated with poor outcomes to infection. B-cell responses develop in response to SARS-CoV2, as demonstrated by the serum IgM and IgG antibody responses that are seen within 2 weeks of infection, as is also seen with MERS-CoV and SARS-CoV1(65). Antibodies are directed primarily against the S and N proteins, and the ACE2-binding site is quite immunogenic, resulting in robust neutralising antibody responses(52).
Effects of vitamin A on adaptive immunityAs discussed above, dendritic cells are a key bridge from innate to adaptive immunity during infection and immunisation. Dendritic cells provide the signals necessary to stimulate development of pathogen-appropriate adaptive immune responses. These signals include peptide antigen to provide pathogen-specificity (i.e. recognition of a specific strain of the flu virus by memory T cells), co-stimulation to insure continued proliferation of developing T cells and specific cytokines to steer CD4 T-cell development towards the pathogen-appropriate Th1, Th2, Th17, Treg and CD4+ follicular-helper T phenotypes. In addition, a fourth signal that is often provided by dendritic cells, particularly in the intestinal and other mucosal-associated immune tissues, is retinoic acid(66), the production of which is diminished during VA deficiency. Retinoic acid affects several aspects of T-cell development, including modification of T-cell phenotype development and mucosal homing of lymphocytes. These effects have recently been reviewed(12). They are pleiotropic and depend on modifying factors (e.g. the local cytokine environment), but several main points are clear.
Perhaps, the most consistent and biologically important effect of retinoic acid produced by dendritic cells, and a principal reason for retinoic acid production being constitutive in mucosal dendritic cells, is that retinoic acid induces transcription of two mucosal-homing molecules on lymphocytes, chemokine receptor 9 and α4β7 integrin. Thus, memory T cells, B cells and antibody-producing plasma cells which develop in the intestine or respiratory tract (both are initiating sites of the common mucosal immune response) will preferentially recirculate to these sites, whereas T cells developing from systemic lymph nodes are less likely to do so(66). Mucosal infections often trigger secretory IgA responses, and the plasma cells responsible for secreting IgA are found at submucosal sites due to the imprinting of chemokine receptor 9 and α4β7 integrin expression. This mechanism may explain why VA deficiency sharply reduces the secretory IgA response to influenza A virus infection in the nasal mucosa of mice(67). Furthermore, impaired IgA antibody and infrequent virus-specific CD8+ T response following respiratory virus infection induced a cytokine storm in the upper respiratory tract of VA-deficient mice(68). Retinoic acid also enhances intestinal homing of other cell types, including Treg cells(69).
Second, VA deficiency has contrasting effects on Th1/Th2 development, with deficiency not affecting or sometimes enhancing Th1 development (via increased IL-12 production, as discussed above) but dampening Th2 development, particularly during the early stages of phenotype commitment immediately after antigenic stimulation. In contrast, retinoic acid, or high-level dietary VA, can enhance type 2 immunity(70). However, retinoic acid, at a somewhat later stage in development of Th1 memory cells, can help stabilise phenotype commitment to the Th1 lineage(71). Similarly, effects of VA on CD8+ cytotoxic T-lymphocyte development, a key component of type 1, anti-viral immunity are mixed(12). Using model viral infections to explore the effects of VA deficiency has shown no impairment of clearance of acute influenza A virus infection in mice, a slight enhancement of some aspects of the type 1 response squamous metaplasia during recovery(31). Using chronic lymphocytic choriomeningitis virus infection in mice also showed an elevated type 1 response, more severe pathological changes, higher virus load and increased mortality, effects which were reversed with retinoic acid(72).
Third, VA regulates the balance between Th17 and Treg development. Both cell types often develop in the intestinal lymphoid tissue (as well as at other sites) with Treg cells being predominant when inflammation is minimal and constitutive TGF-β and retinoic acid production bias memory T-cell development towards a Treg phenotype which is appropriated for antigens derived from food and commensal bacteria. However, when inflammation develops, IL-6 is produced and this shifts the balance towards Th17. During VA deficiency, Treg development is diminished because retinoic acid production is reduced(55).
Fourth, retinoic acid affects B cell development, and VA deficiency decreases the antibody response to T-cell-dependent antigens, in particular(12). While VA deficiency in mice slightly enhances type 1-related IgG2a antibody responses, the IgG1 response is diminished and, in particular, the IgA response is specifically diminished because retinoic acid can enhance class-switching to IgA(73,74) .
Contribution of the immune system to COVID-19 pathogenesisApproximately 5 % of COVID-19 patients, many with underlying conditions such as CVD, diabetes mellitus and obesity, develop severe disease with a high risk of death. Male sex and age above 65 years are also risk factors(6). The pathogenesis of severe disease involves excessive activation of the immune system that can result in immunopathology that increases disease severity, as has been reviewed recently(52,75–77) . During the initial innate immune response, a robust IFN type I/III response appears to predict a milder course of disease, presumably by controlling initial virus replication and allowing development of the adaptive response. However, patients with severe disease are more likely to have a lower initial IFN type I/II response (though it may be elevated later) which may result in a failure to control initial viral replication. Prolonged viral replication then appears to result in high-level production of pro-inflammatory cytokines (including TNF-α, IL-1, IL-6 and many others), likely produced by activated macrophages but perhaps by other innate cells such as neutrophils that may be attracted to the lungs. This local hyper-inflammation may dampen development of adaptive immunity and in patients with severe disease lymphopenia, including low levels of T cells, is pronounced. During this ‘cytokine storm’ with activation of innate immune cells, a system response develops that includes elevation of acute phase proteins, particularly CRP, and activation of the complement system which may result in vascular leakage as well as diffuse intravascular coagulation which can cause organ damage thus increasing disease severity. Developing therapeutic interventions to dampen this immune-mediated pathology is a high priority of current research. Potential drug targets include the NF-κB pathway to attenuate TNF-α and IL-6 expression, the JAK/STAT signalling pathway and the sphingosine-1-phosphate receptor 1 pathway(78). The JAK/STAT and sphingosine-1-phosphate receptor 1 signalling pathways are of particular interest since SARS-CoV2 potential down-regulation of ACE2 expression could result in over-production of angiotensin II via the related ACE enzyme, leading to enhanced IL-6 production. JAK/STAT inhibitors such as Baricitinib or sphingosine-1-phosphate receptor 1 receptor agonists such as Fingolimod could attenuate the cytokine storm(78).
Possible implications of vitamin A deficiency for immune response to SARS-CoV2The effects of VA in the immune system discussed above have several possible implications for individuals with VA deficiency infected by SARS-CoV2. First, VA deficiency is not likely to impair a type 1 anti-viral response to SARS-CoV2, but could increase the severity of type 1 inflammation and tissue damage in the lungs following viral infection. Second, after clearance of SARS-CoV2 infection, VA deficiency could impair repair of damaged alveolar pneumocytes and airway epithelium. Third, protective immunity to SARS-CoV2 could be impaired by VA deficiency, particularly the mucosal IgA response, which could be important in resistance to reinfection, though development of memory Th1 and CD8+ cytotoxic T-lymphocyte response might also be affected.
Effect of infections on vitamin A metabolism and statusVA is absorbed in the intestine with high efficiency in healthy individuals (>80 %) and mainly stored in the liver (>90 %), with smaller amounts stored in intestine, kidney, adipose tissue and the lung(79). Release of serum VA bound to retinol-binding protein (RBP4) is homoeostatically controlled and does not change over a wide range of liver reserves(79). During infection, the acute protein response increases the synthesis of hepatic inflammatory cytokines, but at the same time reduces the release of negative acute phase proteins such as RBP4. The subsequent reduction of holo-RBP4 (retinol bound to apo-RBP4) causes a decline in circulating VA even before the acute-phase proteins CRP and AGP have reached peak concentrations(80). Reduced serum retinol and RBP4 concentrations could potentially overestimate the prevalence of VA deficiency in populations with high levels of inflammation(81). Severe infections have also shown to reduce food intake(80), impair absorption by 20–30 %(15), increase metabolic requirements or catabolic losses(80) and result in substantial urinary losses(17,82) . Importantly, respiratory infections combined with low dietary VA intake could deplete liver VA stores in COVID-19 patients suffering from pneumonia to levels associated with VA deficiency, since urinary losses of >1000 μg retinol/d have been observed in 24 % of ICU patients with pneumonia and sepsis, representing a higher amount than the RDA for VA(82). We estimate that this level of urinary loss, combined with a postulated decrease in intake of approximately 50 % of the RDA for women hospitalised with COVID-19, could lead to a loss of approximately 1350 μg/d from liver stores. Such losses would lead to deficient liver stores (<20 μg/g) in approximately 3 weeks for a 61 kg women with relatively low pre-existing liver stores (40 μg/g; estimated liver weight of 2·4 % of body weight). Using data on estimated liver stores among US women of 129 (sd 89) μg/g who consumed high levels of VA (a mean intake of 173 (sd 111) % of the RDA of 700 μg retinol)(83), we estimate that approximately 16 % of women (those at least one sd below the mean) in a population with similarly high intake would have reserves of 40 μg/g or less. In populations consuming lower levels of VA, the percentage of individuals at risk for systemic VA deficiency would be higher. It is important to note however that these systemic effects of VA are different to tissue-specific VA deficiency that could develop following COVID-19 infection (Fig. 1). First, pulmonary fibrosis induced by SARS-CoV2 is characterised by the conversion of lipofibroblasts to activated myofibroblasts that excessively deposit extracellular matrix proteins and lose their retinoid stores(45,48) . Second, inflammation induced reduction of retinoic acid uptake and metabolism to polar metabolites(80) could affect the ability of the lung to regenerate from pulmonary fibrosis, since lipofibroblasts rely on retinoids to initiate, coordinate and regulate alveolar septal eruption and alveolo-genesis(45). Third, the ability to deliver retinoids to the lung is compromised during severe infection due to the decline in circulating VA(80). Nutritionists have long recognised that acute infection exacerbates the risk of malnutrition, as there is potential to fall into a ‘vicious circle’ of acute illness, deficiency and susceptibility to secondary infection. However, the timing of VA supplementation during SARS-CoV2 infection and the recovery period may be critical, as early intervention during the infectious period may be contraindicative due to its impact on the ACE2 receptor(37,38) , but intervention during the recovery period may be critical to address the potentially developing tissue-specific VA deficiency and its effects on the respiratory immune response. Importantly, VA supplementation may not be beneficial depending on age and co-morbidity, since VA supplementation can increase the risk of acute respiratory tract infections in children with adequate VA status(84) and can delay recovery from community-acquired pneumonia in children(85) and respiratory syncytial virus infection in infants(86). However, VA supplementation was associated with improved recovery from Ebola virus infection in adults(87) and can have beneficial effects in obesity by correcting tissue VA deficiency, altering lung immune cell composition, improving vaccine responses and assisting in respiratory virus clearance(88). In particular, high-dose VA supplementation during recovery from measles has been shown to decrease mortality and improve recovery, including decreasing the severity of secondary bacterial pneumonia(89). Delivering VA through inhalation may be more efficient to address the immunological lesions in VA-deficient individuals(90,91) particularly since inflammation also reduces retinoic acid uptake and metabolism to polar metabolites(80).

Fig. 1. Overview of potential interactions between vitamin A and COVID-19. SARS-CoV2 uses angiotensin-converting enzyme 2 (ACE2) for receptor-mediated cell entry, leading to ACE2 down-regulation and a deregulation of the renin–angiotensin system. Viral RNA triggers the production of CD4+ T-helper type 1 cells and cytokines, including TNF-α, IL-1, IL-6 and IL-18. In the lung, SARS-CoV2 can lead to severe acute respiratory syndrome due to extensive pulmonary fibrosis promoted by enhanced lipofibroblast–myofibroblast transition. Since lipofibroblasts rely on retinoids to initiate, coordinate and regulate alveolar septal eruption and alveolo-genesis, the loss of retinoids during the virus-induced lipofibroblast–myofibroblast transition could impair the ability of the lung to repair damaged epithelial surfaces, potentially leading to long-lasting scarring, lung fibrosis and reduced pulmonary capacity, which could manifest into a ‘long COVID’ effect. Treatment of COVID-19 patients with dexamethasone could further increase localised vitamin A deficiency through the reduction of retinoid-binding proteins and receptors. On the other hand, SARS-CoV2 could also lead to systemic vitamin A deficiency through a combination of increased urinary losses, reduced intake and absorption and increased utilisation. The effects of local and systemic vitamin A deficiency have been shown to reduce the ability of the immune system to maintain healthy respiratory and intestinal epithelial barriers, activity and numbers of type 1 innate lymphoid cells and secretory IgA responses to virus infection. This vicious cycle of increased vitamin A deficiency and decreased regulatory and protective immunity impairs recovery and is likely to increase morbidity and mortality.
Interaction of vitamin A with olfactory functionSARS-CoV2 causes olfactory and/or gustatory dysfunctions in COVID-19 patients(92–95). A recent meta-analysis of eight studies confirmed that patients with COVID-19 had significantly higher risks of developing olfactory and/or gustatory dysfunctions compared with normal subjects (OR of 65·9) or patients with acute respiratory infection without detectable virus (OR of 11·3)(94). On average, 50 % of COVID-19 patients suffered from olfactory and/or gustatory dysfunctions(94), although olfactory impairment was reported to vary between 4·8 % in China, 68 % in the USA and 85·6 % in Europe(93). The mean time from olfactory loss to recovery onset was reported as 12 d, with complete olfactory recovery in only 51 % of patients(93). Viral infections are a common cause of olfactory dysfunction(96), although the pathogenesis of sensory loss after viral infections is not well characterised(92,95) . It is assumed that the nasal epithelium and olfactory bulb could be critical in the pathological development of COVID-19, with mild outcomes of COVID-19 leading to localised effects with mild effects on olfactory function. Interestingly, the effects of SARS-CoV2 on chronic olfactory impairment increase with age, affecting up to 50 % of people aged ≥65 years and >80 % of people aged ≥80 years(92).
VA supplementation may play an important role in the regeneration of olfactory receptor neurons, particularly since retinoic acid signalling is involved in neuronal regeneration(97). Indeed, a recent retrospective cohort analysis in patients with post-infectious olfactory dysfunction indicated that topically applied VA at a dose of 10 000 IU/d for 8 weeks improved clinical outcome(98). This beneficial effect of VA to improve olfactory dysfunction is supposedly due to increased local concentration of VA at the olfactory neuroepithelium(98). Thus, similarly for treating VA deficiency in the lung, topical administration of VA may increase bioavailability and reduce toxic side effects of high-dose systemic VA therapy needed to overcome localised VA deficiency(91,98,99) . However, since no studies have investigated the benefit of topical application of VA in COVID-19 patients suffering from olfactory dysfunction, prospective, double-blind and placebo-controlled trials are needed to confirm if VA therapy would be of benefit to those patients that struggle to recover from olfactory impairment.
Interaction of vitamin A with current SARS-CoV2 medicationThe RECOVERY trial results demonstrated that low-dose dexamethasone reduced the death rate of patients that require respiratory support(3). However, although corticosteroids have anti-inflammatory, antioxidant, pulmonary vasodilator and anti-edematous effects(100), their impact on limiting immune responses might counteract viral clearance(101). Previous studies associate the use of corticosteroid in SARS, MERS and influenza with a higher risk of a delayed viral clearance and long-term complications in survivors(102). Furthermore, dexamethasone decreases alveolar numbers, and this effect on alveolar architecture can be long term or permanent(18). The drug also reduces retinoid-binding proteins and receptors in mice(18), which potentially could affect the metabolism of VA in affected tissues. Since chronic nutritional VA deficiency can result in pulmonary fibrosis(14), long-term complications in survivors after dexamethasone treatment could include lung tissue scaring due to medication-induced VA deficiency. On the other hand, supplementation with a nutrient-metabolite combination containing VA and retinoic acid in a 10:1 molar ratio (VARA) was effective in increasing the concentration of VA to a level that was not antagonised by dexamethasone, probably since the VARA-induced lung tissue VA accumulation compensated the VA loss caused by dexamethasone(103). Importantly, the delivery of retinoids to the lung tissue determines whether or not the negative side effects of dexamethasone on lung VA levels can be overcome. Treatment with retinoic acid was shown to reverse pulmonary emphysema(104), but caused significant toxic side effects(105). To obtain biologically effective levels in the lung, inhalation of retinoids rather than oral administration may be a better approach(91), particularly since this approach induces cellular retinol-binding protein 1 protein expression which was shown to be reduced following dexamethasone treatment(18). However, effectiveness of either VARA or retinoic acid inhalation to optimise lung VA stores would need to be tested in COVID-19-affected individuals who have been treated with dexamethasone.
Vitamin A in resistance to and recovery from infection: relevance to SARS-CoV2 | British Journal of Nutrition | Cambridge Core
Vitamin A in resistance to and recovery from infection: relevance to SARS-CoV2www.cambridge.org
would you be able to summarize the points from that, is vitamin A needed or worsens covid based on that
md_a
Member
- Joined
- Aug 31, 2015
- Messages
- 468
mate whyd you cross everything out with the lines through it
would you be able to summarize the points from that, is vitamin A needed or worsens covid based on that
I tried to post from the phone while I was at work and an error occurred with those cut sentences, and I could not correct due to lack of time.
..........
SARS-CoV2 pathogenesis and effects of vitamin A on the immune system
SARS-CoV2 infection of the respiratory epithelium
Similarly to SARS-CoV1, the cellular infection mechanism of SARS-CoV2 is mainly mediated by the cell-surface receptor angiotensin-converting enzyme 2 (ACE2)(19). SARS-CoV2 uses ACE2 for receptor-mediated cell entry and serine protease transmembrane serine protease 2 (TMPRSS2) for S protein priming(20). Cell entry of SARS-CoV2 can be partially blocked by the clinically proven serine protease inhibitor camostat mesylate, which is active against serine protease transmembrane serine protease 2 (TMPRSS2)(20). Although very low levels of ACE2 protein have been observed in the normal respiratory system(21), the presence of ACE2 protein levels and viral RNA has been confirmed in ciliated epithelial cells, indicating that low-level protein expression in upper airway epithelial cells facilitates infection of ciliated cells, followed by a rapid interferon-induced increase of ACE2 expression in lower airway ciliated and type-2 alveolar epithelial cells, potentially allowing SARS-CoV2 to spread across the respiratory mucosa(22). The cell-surface ACE2 receptor internalises on binding to the SARS-CoV spike protein, leading to ACE2 receptor down-regulation(23,24) . The SARS-CoV spike protein-mediated ACE2 down-regulation could contribute to the severity of lung pathologies such as pulmonary fibrosis and acute respiratory distress syndrome, since the counterbalance of ACE2 on angiotensin II production in the renin–angiotensin system is deregulated, affecting its ability to modulate the innate immune system and to regulate inflammation(19,25,26) . ACE2 knockout mice infected with SARS-CoV1 show reduced pathological alterations in the lung(19). Increased incidence of intravascular coagulation with SARS-CoV2 infection could also be mediated via ACE2, since ACE2 deficiency is associated with up-regulation of putative mediators of atherogenesis, such as cytokines and adhesion molecules, and reduced ACE expression is observed in established atherosclerotic plaques(27). Finally, the observed difference in SARS-CoV2 severity between children and adults could in part be attributed to the lower expression of the SARS-CoV2-targeted receptor ACE2 and serine protease serine protease transmembrane serine protease 2 (TMPRSS2) in nasal and bronchial airways in children compared with adults(28).Vitamin A and epithelial barriers
VA is needed to maintain healthy respiratory and intestinal epithelial barriers(17). VA deficiency may not produce obvious changes in healthy epithelial surfaces, but following viral infection in the intestine, for example, pathological changes to infected epithelial surfaces were found to be much greater in VA-deficient than in control mice(29). In the respiratory tract, VA deficiency also increases epithelial damage and impairs recovery, sometimes leading to squamous metaplasia in alveoli and airways, following noxious exposures such as ozone treatment(30) and infection with influenza virus(31). Sputum (mucus) also provides a solid physical barrier to pathogens(32) and contains many of the macromolecules of the innate immune system(33). Sputum is a mixture of oligomeric mucins, MUC2, MUC5AC and MUC5B which are synthesised and secreted by the differentiated mucociliary epithelium(34). Regulation of mucin production is induced by all-trans retinoic acid via retinoic acid receptor alpha as the major retinoid receptor subtype(35). Importantly, moderate VA supplementation, but not high-dose VA supplementation or VA deficiency, improves mucin secretion by regulating the gene expression of cytokines and epithelial growth factors(36).The VA metabolite all-trans retinoic acid has also been shown to up-regulate the expression of ACE2(37,38) , resulting in the reduction of blood pressure and the attenuation of myocardial damage in spontaneously hypertensive rats(37). Increased levels of ACE2 after VA supplementation could have two effects: 1) it could increase the risk of SARS-CoV2 infection at the time of virus exposure(39), particularly in individuals who have an adequate VA status; or 2) it could reduce the risk of sympathetic over-activation seen in severe SARS-CoV2 infection, obese and diabetic patients(40–42) through VA supplementation that activates the ACE2-Ang 1–7-Mas axis.
VA is also important during embryonic lung development(43); thus, the role in tissue repair in postnatal life is not surprising. A recent review highlights the potential role of VA and retinoic acid as possible therapeutic interventions to prevent pulmonary fibrosis following lung injury(44), suggesting a role for VA in recovery from severe COVID-19 disease. Lipofibroblasts are the retinoid storage cells of the lung, similar to stellate cells of the liver(18), and contain many components of the retinoid signalling pathway including receptors and binding proteins(45). Importantly, the defining characteristic of hepatic fibrosis is the loss of VA from stellate cells(46). Similarly, chronic nutritional VA deficiency results in pulmonary fibrosis through decreased alveolar septation, squamous metaplasia of the respiratory epithelium and a thickening of the alveolar basement membrane followed by an increase in collagens and a deposition of ectopic collagen fibrils(14). Pulmonary fibrosis is characterised by conversion of lipofibroblasts to FGF10-expressing myofibroblasts localised to αSMA expressing cells, down-regulation of PPARγ and activation of TGF-β signalling(47). PPARγ activation reverts fibrosis in both the lung and the liver through inhibition of αSMA, type I collagen and TGF-β expression(46,48) . The beneficial effects of retinoids on pulmonary fibrosis may be through activation of the retinoid X receptor via 9-cis-retinoic acid(49).
Innate immune response to SARS-CoV2 infection
The immune response to respiratory viral infections, including coronaviruses, is initiated by barrier defences at the site of initial virus replication(50). SARS-CoV2 infects ciliated epithelial cells in the upper respiratory tract and alveolar type II pneumocytes in the lower respiratory tract via its cellular receptor ACE2(51). SARS-CoV2 thus first engages the innate immune system in respiratory epithelial cells and adjacent macrophages. Viral RNA in the cytoplasm or endosomes is recognised by pattern-recognition receptors including RIG-1, TLR3, TLR7 and TLR9. These receptors trigger transcription of type I and type III interferon (IFN) which act via cellular receptors to induce anti-viral programmes in infected and adjacent cells that render these cells refractory to viral infection. However, coronaviruses, including SARS-CoV2, can act on these pathways to block induction of anti-viral activity, which may be a factor in the development of more severe diseases. In addition to type I and III IFN, pattern-recognition receptor activation triggers production of other cytokines, including TNF-α, IL-1, IL-6 and IL-18 by innate immune cells, particularly macrophages. These cytokines help initiate inflammation, including recruitment of other immune cells to the site of initial infection. Innate lymphoid cells found at tissue sites are often involved in the initial response to viral (and other) infections, but their role in SARS-CoV2 infection is uncertain(52,53) .Effects of vitamin A on innate immunity
Many cells of the innate immune system, including innate lymphoid cells, macrophages and granulocytes, are affected by VA. Type 1 innate lymphoid cells, including natural killer (NK) cells, play a role in responses to viral infection and their activity and number in peripheral blood is decreased by VA deficiency(54), while retinoic acid treatment can also dampen activity(55). Neutrophil and macrophage function is also directly affected by VA. Phagocytic and bacterial-killing activity of these cells is impaired by VA deficiency, leading to decreased resistance to some infections, though production of the CD4+ T-helper type 1 (Th1)-promoting cytokine IL-12 can be increased, causing increased type 1 inflammation(17). Ex vivo and animal studies show that treatment of macrophages with retinoic acid can have anti-inflammatory effects, dampening production of IL-12 and pro-inflammatory cytokines such as TNF-α, as well as increasing production of the regulatory cytokine IL-10(55). Other effects on macrophage phenotype are also caused by retinoic acid. For example, in Leishmania infection of mice, retinoic acid treatment diminishes the development of M1 macrophages, a component of protective type 1 immunity in this model system, and increases the development of M2 macrophages and type 2 immunity, which is not protective. Thus, retinoic acid intervention in this system decreases resistance to infection(56). Conversely, in Schistosoma mansoni infection of mice, VA deficiency exacerbates disease due to excessive inflammation and tissue damage, while retinoic acid treatment increases conversion of macrophages into a tissue-resident phenotype that dampens inflammation and allows control of infection and enhancement of tissue repair(57). While many of the effects of retinoic acid on innate immune cells can be seen as anti-inflammatory, a lesson from these and other studies is that intervention with retinoic acid during infections can have unpredictable effects on the course of a particular infection depending on the specifics of the immune response that are responsible for pathogen clearance. This topic has been discussed in a recent review(58).Effects of vitamin A on adaptive immunity
As discussed above, dendritic cells are a key bridge from innate to adaptive immunity during infection and immunisation. Dendritic cells provide the signals necessary to stimulate development of pathogen-appropriate adaptive immune responses. These signals include peptide antigen to provide pathogen-specificity (i.e. recognition of a specific strain of the flu virus by memory T cells), co-stimulation to insure continued proliferation of developing T cells and specific cytokines to steer CD4 T-cell development towards the pathogen-appropriate Th1, Th2, Th17, Treg and CD4+ follicular-helper T phenotypes. In addition, a fourth signal that is often provided by dendritic cells, particularly in the intestinal and other mucosal-associated immune tissues, is retinoic acid(66), the production of which is diminished during VA deficiency. Retinoic acid affects several aspects of T-cell development, including modification of T-cell phenotype development and mucosal homing of lymphocytes. These effects have recently been reviewed(12). They are pleiotropic and depend on modifying factors (e.g. the local cytokine environment), but several main points are clear.Perhaps, the most consistent and biologically important effect of retinoic acid produced by dendritic cells, and a principal reason for retinoic acid production being constitutive in mucosal dendritic cells, is that retinoic acid induces transcription of two mucosal-homing molecules on lymphocytes, chemokine receptor 9 and α4β7 integrin. Thus, memory T cells, B cells and antibody-producing plasma cells which develop in the intestine or respiratory tract (both are initiating sites of the common mucosal immune response) will preferentially recirculate to these sites, whereas T cells developing from systemic lymph nodes are less likely to do so(66). Mucosal infections often trigger secretory IgA responses, and the plasma cells responsible for secreting IgA are found at submucosal sites due to the imprinting of chemokine receptor 9 and α4β7 integrin expression. This mechanism may explain why VA deficiency sharply reduces the secretory IgA response to influenza A virus infection in the nasal mucosa of mice(67). Furthermore, impaired IgA antibody and infrequent virus-specific CD8+ T response following respiratory virus infection induced a cytokine storm in the upper respiratory tract of VA-deficient mice(68). Retinoic acid also enhances intestinal homing of other cell types, including Treg cells(69).
Second, VA deficiency has contrasting effects on Th1/Th2 development, with deficiency not affecting or sometimes enhancing Th1 development (via increased IL-12 production, as discussed above) but dampening Th2 development, particularly during the early stages of phenotype commitment immediately after antigenic stimulation. In contrast, retinoic acid, or high-level dietary VA, can enhance type 2 immunity(70). However, retinoic acid, at a somewhat later stage in development of Th1 memory cells, can help stabilise phenotype commitment to the Th1 lineage(71). Similarly, effects of VA on CD8+ cytotoxic T-lymphocyte development, a key component of type 1, anti-viral immunity are mixed(12). Using model viral infections to explore the effects of VA deficiency has shown no impairment of clearance of acute influenza A virus infection in mice, a slight enhancement of some aspects of the type 1 response squamous metaplasia during recovery(31). Using chronic lymphocytic choriomeningitis virus infection in mice also showed an elevated type 1 response, more severe pathological changes, higher virus load and increased mortality, effects which were reversed with retinoic acid(72).
Third, VA regulates the balance between Th17 and Treg development. Both cell types often develop in the intestinal lymphoid tissue (as well as at other sites) with Treg cells being predominant when inflammation is minimal and constitutive TGF-β and retinoic acid production bias memory T-cell development towards a Treg phenotype which is appropriated for antigens derived from food and commensal bacteria. However, when inflammation develops, IL-6 is produced and this shifts the balance towards Th17. During VA deficiency, Treg development is diminished because retinoic acid production is reduced(55).
Fourth, retinoic acid affects B cell development, and VA deficiency decreases the antibody response to T-cell-dependent antigens, in particular(12). While VA deficiency in mice slightly enhances type 1-related IgG2a antibody responses, the IgG1 response is diminished and, in particular, the IgA response is specifically diminished because retinoic acid can enhance class-switching to IgA(73,74) .
Effect of infections on vitamin A metabolism and status
VA is absorbed in the intestine with high efficiency in healthy individuals (>80 %) and mainly stored in the liver (>90 %), with smaller amounts stored in intestine, kidney, adipose tissue and the lung(79). Release of serum VA bound to retinol-binding protein (RBP4) is homoeostatically controlled and does not change over a wide range of liver reserves(79). During infection, the acute protein response increases the synthesis of hepatic inflammatory cytokines, but at the same time reduces the release of negative acute phase proteins such as RBP4. The subsequent reduction of holo-RBP4 (retinol bound to apo-RBP4) causes a decline in circulating VA even before the acute-phase proteins CRP and AGP have reached peak concentrations(80). Reduced serum retinol and RBP4 concentrations could potentially overestimate the prevalence of VA deficiency in populations with high levels of inflammation(81). Severe infections have also shown to reduce food intake(80), impair absorption by 20–30 %(15), increase metabolic requirements or catabolic losses(80) and result in substantial urinary losses(17,82) . Importantly, respiratory infections combined with low dietary VA intake could deplete liver VA stores in COVID-19 patients suffering from pneumonia to levels associated with VA deficiency, since urinary losses of >1000 μg retinol/d have been observed in 24 % of ICU patients with pneumonia and sepsis, representing a higher amount than the RDA for VA(82). We estimate that this level of urinary loss, combined with a postulated decrease in intake of approximately 50 % of the RDA for women hospitalised with COVID-19, could lead to a loss of approximately 1350 μg/d from liver stores. Such losses would lead to deficient liver stores (<20 μg/g) in approximately 3 weeks for a 61 kg women with relatively low pre-existing liver stores (40 μg/g; estimated liver weight of 2·4 % of body weight). Using data on estimated liver stores among US women of 129 (sd 89) μg/g who consumed high levels of VA (a mean intake of 173 (sd 111) % of the RDA of 700 μg retinol)(83), we estimate that approximately 16 % of women (those at least one sd below the mean) in a population with similarly high intake would have reserves of 40 μg/g or less. In populations consuming lower levels of VA, the percentage of individuals at risk for systemic VA deficiency would be higher. It is important to note however that these systemic effects of VA are different to tissue-specific VA deficiency that could develop following COVID-19 infection (Fig. 1). First, pulmonary fibrosis induced by SARS-CoV2 is characterised by the conversion of lipofibroblasts to activated myofibroblasts that excessively deposit extracellular matrix proteins and lose their retinoid stores(45,48) . Second, inflammation induced reduction of retinoic acid uptake and metabolism to polar metabolites(80) could affect the ability of the lung to regenerate from pulmonary fibrosis, since lipofibroblasts rely on retinoids to initiate, coordinate and regulate alveolar septal eruption and alveolo-genesis(45). Third, the ability to deliver retinoids to the lung is compromised during severe infection due to the decline in circulating VA(80). Nutritionists have long recognised that acute infection exacerbates the risk of malnutrition, as there is potential to fall into a ‘vicious circle’ of acute illness, deficiency and susceptibility to secondary infection. However, the timing of VA supplementation during SARS-CoV2 infection and the recovery period may be critical, as early intervention during the infectious period may be contraindicative due to its impact on the ACE2 receptor(37,38) , but intervention during the recovery period may be critical to address the potentially developing tissue-specific VA deficiency and its effects on the respiratory immune response. Importantly, VA supplementation may not be beneficial depending on age and co-morbidity, since VA supplementation can increase the risk of acute respiratory tract infections in children with adequate VA status(84) and can delay recovery from community-acquired pneumonia in children(85) and respiratory syncytial virus infection in infants(86). However, VA supplementation was associated with improved recovery from Ebola virus infection in adults(87) and can have beneficial effects in obesity by correcting tissue VA deficiency, altering lung immune cell composition, improving vaccine responses and assisting in respiratory virus clearance(88). In particular, high-dose VA supplementation during recovery from measles has been shown to decrease mortality and improve recovery, including decreasing the severity of secondary bacterial pneumonia(89). Delivering VA through inhalation may be more efficient to address the immunological lesions in VA-deficient individuals(90,91) particularly since inflammation also reduces retinoic acid uptake and metabolism to polar metabolites(80).
nteraction of vitamin A with olfactory function
SARS-CoV2 causes olfactory and/or gustatory dysfunctions in COVID-19 patients(92–95). A recent meta-analysis of eight studies confirmed that patients with COVID-19 had significantly higher risks of developing olfactory and/or gustatory dysfunctions compared with normal subjects (OR of 65·9) or patients with acute respiratory infection without detectable virus (OR of 11·3)(94). On average, 50 % of COVID-19 patients suffered from olfactory and/or gustatory dysfunctions(94), although olfactory impairment was reported to vary between 4·8 % in China, 68 % in the USA and 85·6 % in Europe(93). The mean time from olfactory loss to recovery onset was reported as 12 d, with complete olfactory recovery in only 51 % of patients(93). Viral infections are a common cause of olfactory dysfunction(96), although the pathogenesis of sensory loss after viral infections is not well characterised(92,95) . It is assumed that the nasal epithelium and olfactory bulb could be critical in the pathological development of COVID-19, with mild outcomes of COVID-19 leading to localised effects with mild effects on olfactory function. Interestingly, the effects of SARS-CoV2 on chronic olfactory impairment increase with age, affecting up to 50 % of people aged ≥65 years and >80 % of people aged ≥80 years(92).VA supplementation may play an important role in the regeneration of olfactory receptor neurons, particularly since retinoic acid signalling is involved in neuronal regeneration(97). Indeed, a recent retrospective cohort analysis in patients with post-infectious olfactory dysfunction indicated that topically applied VA at a dose of 10 000 IU/d for 8 weeks improved clinical outcome(98). This beneficial effect of VA to improve olfactory dysfunction is supposedly due to increased local concentration of VA at the olfactory neuroepithelium(98). Thus, similarly for treating VA deficiency in the lung, topical administration of VA may increase bioavailability and reduce toxic side effects of high-dose systemic VA therapy needed to overcome localised VA deficiency(91,98,99) . However, since no studies have investigated the benefit of topical application of VA in COVID-19 patients suffering from olfactory dysfunction, prospective, double-blind and placebo-controlled trials are needed to confirm if VA therapy would be of benefit to those patients that struggle to recover from olfactory impairment.
Summary
Traditionally, VA plays a very important role in the response of the immune system to infection, and this role may be particularly important in the development of a protective immune response after a SARS-CoV2 infection has occurred. This stresses the need for an adequate VA status prior to infection in order to support the immune system in its ability to deal with the inflammatory response due to SARS-CoV2 via a range of mechanisms that have been summarised in this review. However, in spite of an adequate a priori VA reserve, SARS-CoV2 infection itself may diminish VA stores. Such an infection-induced VA deficiency may impair the ability of the lung to repair damaged epithelial surfaces, potentially leading to long-lasting scarring, lung fibrosis and reduced pulmonary capacity. Thus, VA supplementation during recovery may be needed even though VA supplementation early in infection may not have been indicated due to a lack of VA deficiency prior to infection. In addition, earlier VA supplementation may have unpredictable effects for the following reasons: First, it is plausible that VA supplementation at the time of SARS-CoV2 exposure would increase the risk or severity of an infection since the cellular infection mechanism of SARS-CoV2 is mainly mediated by ACE2 which can be up-regulated by retinoic acid, as discussed above. Second, however, activation of ACE2 in infected patients could reduce the risk of sympathetic over-activation seen in severe SARS-CoV2 infection, and in particular in obese and diabetic COVID-19 patients. Topical application of VA may be beneficial to patients suffering from post-infectious olfactory dysfunction, since VA supplementation may play an important role in the regeneration of olfactory receptor neurons. Finally, there could be a beneficial use of VA as a co-medication in severe SARS-CoV2-affected patients who are treated with corticosteroids, particularly since dexamethasone was shown to negatively affect VA metabolism in lung tissues. It is plausible to assume that VA deficiency in at risk population groups would increase the risk of severe COVID-19 infection, but data to confirm this are currently missing. Importantly, COVID-19 has doubled the number of people in lower- and middle-income countries facing acute food insecurity and is predicted to reduce the coverage of VA supplementation programmes to VAD-affected populations between 15 and 50 %(106). Since the risk of SARS-CoV2 infection is low in under 5-year-olds, and the benefit of VA supplementation outweighs the risk of developing a severe COVID-19 infection due to increased VA intake, VA supplementation programmes should continue for this age group.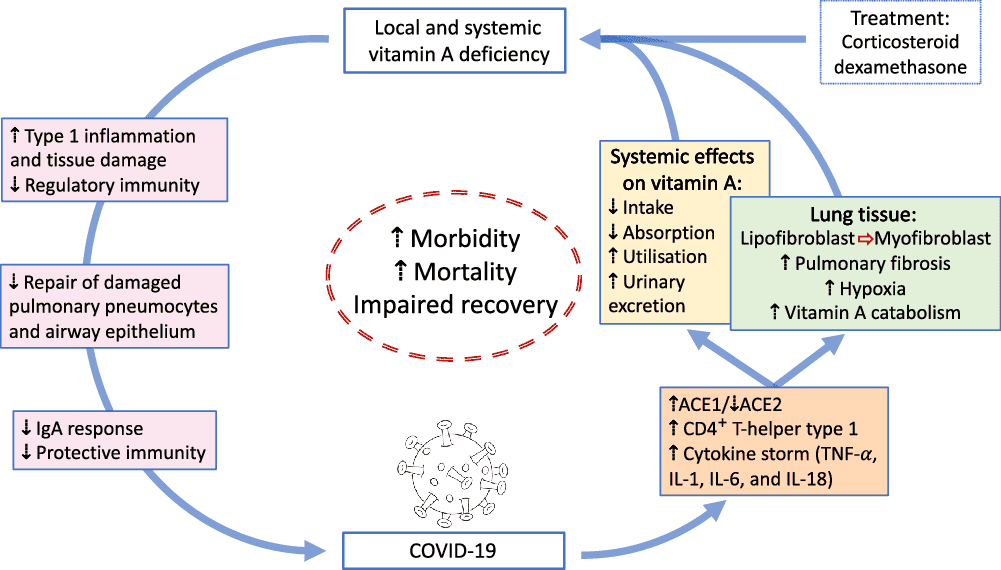
Vitamin A in resistance to and recovery from infection: relevance to SARS-CoV2 | British Journal of Nutrition | Cambridge Core
Vitamin A in resistance to and recovery from infection: relevance to SARS-CoV2 - Volume 126 Issue 11
Amazoniac
Member
BA8C: trans-b-apo-8'-Carotenal
A constipated and inflammed gut would be a situation for these toxins to reach troubling concentrations in susceptible conditions.
I wonder if this can be another reason for their therapeutic value in cancer.
Don't listen to any idiots defending the VA here. Imho, "vitamin A" is the second (if not the first) fraud of big pharma on the scale, after PUFA. I didn't believe it either at first, but after trying Grant's diet on myself and studying the arguments of both sides, I realized who was right.I'm so lost, I don't know which way to go. Should I try vitamin A elimination diet or do I go for real animal based vitamin A way?
All of my health problems started when I was at the end of my second heavy accutane course. I started having really bad cranial pressure, my face started to have hot flushes and my back was sweaty all the time even though it was really chilly outside. I also took Ast-ss brand multivitamin which had quite high vitamin A content. I have never really eaten any real animal based vitamin A rich foods, my A intake has come from supplements.
Chris Masterjohn says that accutane actually causes real vitamin A deficiency and EastWest healing says that vitamin A supplements don't work like real animal based A.
So I really don't know what to do, do I have real animal based vitamin A deficiency or do I have toxicity that only goes away with total A elimination. This is so hard and I'm so tired that I don't even know do I have the strength and will to even find out anymore.

Accutane causes vitamin A deficiency (?)
This comment is in password protected Member area of Masterjohn’s website. It’s the C Vitamins and minerals 101 Q&A area, which you might be able to get …
 ggenereux.blog
ggenereux.blog
Accutane, the "draining" VA, is discussed here.
EMF Mitigation - Flush Niacin - Big 5 Minerals
Similar threads
- Replies
- 24
- Views
- 9K
Low Toxin Videos
Grant Genereaux on Vitamin A, retinol and retinoic acid Toxicity...
- Replies
- 2
- Views
- 1K
Low Toxin Diet
Simple steps to transition to a Low Toxin Diet/Way of Living
- Replies
- 64
- Views
- 9K
- Replies
- 75
- Views
- 15K
- Replies
- 94
- Views
- 16K
- Replies
- 16
- Views
- 4K
Low Toxin Testimonials
Low Vitamin A Diet Testimonials
- Replies
- 87
- Views
- 23K
- Replies
- 201
- Views
- 24K
- Replies
- 13
- Views
- 7K
