so ginger's protease must be very good digestion aid for protein meal.making it more bioavailable.
the quantity of fresh ginger they used is not that much.
Ginger milk curd - Wikipedia
Ginger milk curd

With only 3 ingredients you can make a tasty gel within minutes that can carry the weight of a spoon! Notice how elastic the gel is. After the picture was taken the spoon could be removed, leaving the gel intact.
Mechanism of gelling
Ginger milk curd belongs to a large group of foods where enzymes are used to curdle milk (including cheese of course). Traditionally one would use rennet to prepare such a curd. Rennet is found in the stomach of young mammals and is essential as they digest their mothers’ milk. The active enzyme in rennet is chymosin, also known as rennin. This is a proteolytic enzyme, also known as a protease. These enzymes are capable of breaking proteins into smaller fragments.
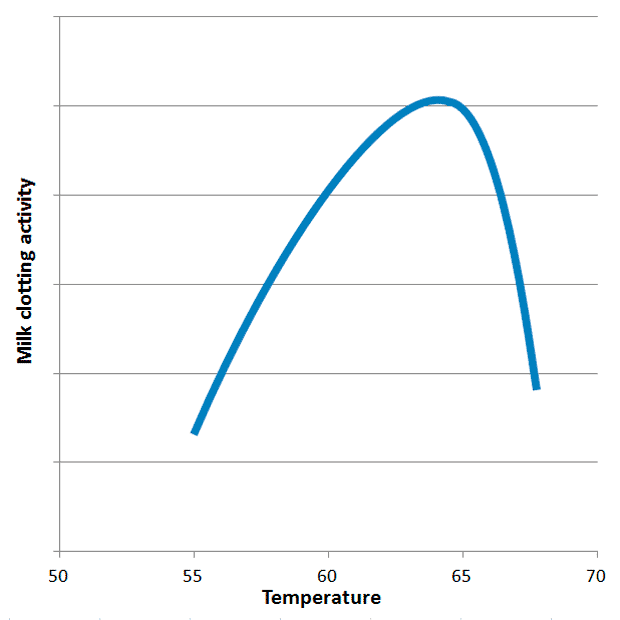
Ginger enters the scene because ginger also contains proteolytic enzymes. The ginger proteases (GP, sometimes called zingipain, EC 3.4.22.67) are very sensitive to temperature. At temperature above 70 °C they are rapidly denatured (=irreversibly destroyed). This explains why so many people fail when trying to make ginger milk curd. The milk clotting activity (MCA) of GP peaks around 63 °C and falls off rapidly above 65 °C and below 60 °C (see figure below). This means one is left with a relatively narrow temperature window of 60-65 °C. GP does show proteolytic activity (PA) outside this window, but this PA is more of a non-specific kind. MCA on the other hand is related to a specific hydrolysis of κ-casein (more on casein in a second, the greek letter κ is pronounced “kappa”). By now you may wonder if it’s possible to make cheese with ginger – and the answer is yes. Scientists have studied several plant extracts and found that ginger, kiwi and melon all contain proteases with a relatively high MCA/PA ratio (albeit not as high as that of chymosin found in rennet). The temperatures for maximum milk clotting activity of kiwi and melon proteases are 40 °C and 70 °C respectively.

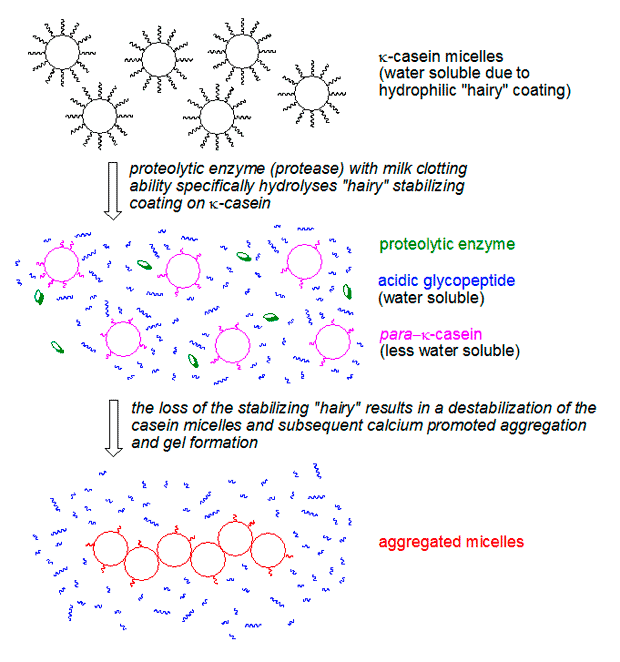
Enzymes are catalysts, the tireless workers responsible for building the gel. But it is casein, a group of proteins found in milk, which is the actual building block of the gel. The casein proteins group together to form large balls known as micelles which are held together by calcium ions. The outside of the micelles is covered with κ-casein which is composed of a water soluble part (an acidic glycopeptide) and another part which is much less soluble in water (para-κ-casein). The water soluble part migrates to the surface and leaves the micelles covered by a “hairy” layer. This layer both keeps the micelles dissolved in water, and prevents the micelles from coming so close to one another that they would coalesce and aggregate.
It is thanks to this “hairy” layer that milk is stable (i.e. does not spontaneously form a gel), and all is well until we add the above mentioned proteases to milk. What chymosin, GP and other proteases do is to cleave of the water soluble part of κ-casein, leaving para-κ-casein behind. Suddenly the micelles can collide, and the calcium present in milk aids in the formation of aggregates. These aggregates of “shaved” micelles make up the actual gel. It all happens within a couple of minutes. The resulting gel is very fragile and easily looses water, a process known as syneresis (see the top picture in the post).

I mentioned above the very narrow temperature window for GP, but one remaining question was whether heating milk to a higher temperature (before cooling to the same temperature window) would be beneficial. It turns out that if milk is heated above 65 °C, the strenght of the resulting gel is reduced. The reason is that the heat causes compounds in the milk (lactoglobulins) to precipitate onto the κ-casein. This interferes with the gel formation. The same is true for milk fat, so skimmed milk is the choice for a stronger gel. Since calcium plays a role in the aggregation of the “shaved” micelles, a higher calcium concentration will also result in a stronger gel.
The ginger juice also deserves a couple of extra words. In freshly squeezed ginger juice the GP has a half-life of 20 min at 30 °C, so in a warm kitchen, half of your enzyme activity is lost 20 min after you have grated and squeezed your ginger. If you leave it another 20 min you’re only left with 25% of the original activity. This means that the ginger juice can’t be prepared in advance or stored unless you use a little trick. The reason for the instability is that ginger also contains another enzyme, polyphenol oxidase (or PPO for short) – the same enzyme that is responsible for the browning of apples (an example of enzymatic browning, as opposed to the Maillard reaction which is an example of non-enzymatic browning). Once the ginger has been grated, PPO attacks phenolic groups yielding ortho-quinones. These in turn can react with the GP enzymes to inactivate them. A well-known trick to prevent the browning of apples is to use ascorbic acid (more commonly known as vitamin C). Ascorbic acid blocks the action of PPO, which in turn prevents the inactivation of the GP enzymes. And the same trick also works for ginger juice. If you need to make ginger juice in advance, just add a pinch of vitamin C (0.2% to be precise).

Ideas for further experimentation
Even though I’ve arrived at a recipe which seems to work fine, there are several claims that remain to be tested – feel free to join in on the experimentation (or share it as an idea for a school science project)!
Mazorra-Manzano, M. A.; Perea-Gutiérrez, T. C.; Lugo-Sánchez, M. E.; Ramirez-Suarez, J. C.; Torres-Llanez, M.; González-Córdova, A. F.; Vallejo-Cordoba, B. “Comparison of the milk-clotting properties of three plant extracts” Food Chem. 2013, 141, 1902-1907. DOI: 10.1016/j.foodchem.2013.05.042
Su, H.-P.; Huang, M.-J.; Wang, H.-T. “Characterization of ginger proteases and their potential as a rennin replacement” J. Sci. Food Agric. 2009, 89, 1178-1185. DOI: 10.1002/jsfa.3572 [free pdf here]
Further reading
Chen, Y.-Y. “Factors Affecting Protease Activity of Ginger and Its Application in Milk Clotting Products”, 2004, Thesis (Language: Chinese).
 --------------
--------------
sounds great especially if used with lactase milk, very easy digestion.
the third study mentions the results are even better than calf" rennet!
Purification, characterization, and milk coagulating properties of ginger proteases. - PubMed - NCBI
Ginger proteases are used as milk coagulants in making a Chinese traditional milk product (Jiangzhinai or Jiangzhuangnai), suggesting their potential as a source of rennet substitute that might be applicable in the modern dairy industry. In this study, ginger proteases were extracted from fresh ginger rhizome by using phosphate buffer and subsequently purified by ion exchange chromatography. Ginger proteases, all with a molecular weight around 31 kDa, were found to exist in 3 forms with isoelectric point values around 5.58, 5.40, and 5.22, respectively. These enzymes had very similar biochemical behavior, exhibiting optimal proteolytic activity from 40 to 60 °C and maximum milk clotting activity at 70 °C. They were capable of hydrolyzing isolated α(S1)-, β-, and κ-casein, of which α(S1)-casein was most susceptible to the enzyme; κ-casein was hydrolyzed with a higher specificity than α(S1)- and β-casein. In addition, the ginger proteases exhibited a similar affinity for κ-casein and higher specificity with increasing temperature. Gel electrophoresis and mass spectra indicated that Ala90-Glu91 and His102-Leu103 of κ-casein were the preferred target bonds of ginger proteases. The milk clotting activity, affinity, and specificity toward κ-casein showed that ginger protease is a promising rennet-like protease that could be used in manufacturing cheese and oriental-style dairy foods.
Characterization of ginger proteases and their potential as a rennin replacement
BACKGROUND: Ginger rhizome (Zingiber officinale Roscoe) contains ginger proteases and has proteolytic activity. Ginger proteases have been used for tenderizing meat but rarely for milk clotting. The purpose of this study was to purify ginger proteases and to research their biochemical characteristics.
RESULTS: The milk clotting activity (MCA) and proteolytic activity (PA) of the proteases was stable after storage at 4 °C for 24 h. The MCA and PA of fresh ginger juice with 0.2% L-ascorbic acid remained stable for 6 days at 4 °C. When under storage at −80 °C for 2 months, the MCA and PA of the fresh ginger juice and acetone precipitate were still high. Two peaks with protease activity were purified from a DEAE FF ion-exchange column; the specific activity (units mg−1 protein) of the MCA (MCSA) and PA (PSA) for the first peak was significantly higher than the second peak (P < 0.05). The protease activity of the ginger proteases was significantly inhibited by E-64, leupeptin, and iodoacetic acid. Zymography results showed that two protease fractions purified from ginger juice with 62 and 82 kDa had a higher PA against α- and β-casein than against κ-casein.
CONCLUSION: The ascorbic acid addition significantly stabilized the MCA and PA of ginger proteases. The protease inhibition test suggested that ginger proteases belonged to the cysteine type. The biochemical characteristics of ginger protease described in this paper can provide useful information for making new milk curd products.
Ginger protease used as coagulant enhances the proteolysis and sensory quality of Peshawari cheese compared to calf rennet
The worldwide increase in cheese consumption combined with a scarcity of rennet as well as ethical concerns have resulted in a global interest for natural milk coagulants from plant sources. In this study, the influence of ginger protease in comparison to calf rennet on the physicochemical, microbiological, and sensory characteristics of Peshawari cheese manufactured from cow’s milk was examined. For most of the physicochemical parameters (fat, protein, lactose, acidity, pH), and the main groups of microorganisms (total viable, enterobacteria, Lactobacilli, and molds and yeasts) investigated, no significant (P > 0.05) differences were observed between the two cheeses made by using different coagulants. However, significantly lower (P < 0.05) levels of moisture and higher levels of soluble nitrogen were observed in the cheese produced by ginger protease compared to that made using calf rennet. The main sensory attributes (appearance, body texture, and flavor) were significantly enhanced (P < 0.05) in Peshawari cheese prepared with ginger protease. Importantly, no bitterness was noted by the sensory panel in the Peshawari cheese made with ginger protease. The results reveal that the ginger protease may have potential application for the manufacture of Peshawari cheese.
------------
its very interesting one of those studies i posted above mentions in high temps the enzymes still active.i was under the impression that the enzyme deactivated and destroyed in higher than 45c...
the quantity of fresh ginger they used is not that much.
Ginger milk curd - Wikipedia
Ginger milk curd
250 mL skimmed milk
18 g fresh *) ginger juice **)
20 g sugar
Combine milk and sugar in a pan and heat carefully to 65 °C. Peel and microplane ginger, and squeeze out the juice. Place juice in a bowl and pour milk into the ginger juice from a height to allow sufficient mixing. Do NOT stir as this will interfere with the gel formation. Leave to set at room temperature. After 5-10 min a gel has formed. The curd may be served immediately or kept in the fridge.
In this recipe I used a milk:ginger juice ratio of 14:1. You can certainly use more ginger juice, but the taste of ginger may then become too powerful. You can also reduce the amount of ginger juice, but this I havent’ tested yet.
*) See comment below regarding the stability of the enzymes. Ginger juice should not be made in advance due to a short half-life in room temperature.
**) 18 g ginger juice = ca. 31 g peeled ginger = ca. 43 g raw ginger.

With only 3 ingredients you can make a tasty gel within minutes that can carry the weight of a spoon! Notice how elastic the gel is. After the picture was taken the spoon could be removed, leaving the gel intact.
Mechanism of gelling
Ginger milk curd belongs to a large group of foods where enzymes are used to curdle milk (including cheese of course). Traditionally one would use rennet to prepare such a curd. Rennet is found in the stomach of young mammals and is essential as they digest their mothers’ milk. The active enzyme in rennet is chymosin, also known as rennin. This is a proteolytic enzyme, also known as a protease. These enzymes are capable of breaking proteins into smaller fragments.
Ginger enters the scene because ginger also contains proteolytic enzymes. The ginger proteases (GP, sometimes called zingipain, EC 3.4.22.67) are very sensitive to temperature. At temperature above 70 °C they are rapidly denatured (=irreversibly destroyed). This explains why so many people fail when trying to make ginger milk curd. The milk clotting activity (MCA) of GP peaks around 63 °C and falls off rapidly above 65 °C and below 60 °C (see figure below). This means one is left with a relatively narrow temperature window of 60-65 °C. GP does show proteolytic activity (PA) outside this window, but this PA is more of a non-specific kind. MCA on the other hand is related to a specific hydrolysis of κ-casein (more on casein in a second, the greek letter κ is pronounced “kappa”). By now you may wonder if it’s possible to make cheese with ginger – and the answer is yes. Scientists have studied several plant extracts and found that ginger, kiwi and melon all contain proteases with a relatively high MCA/PA ratio (albeit not as high as that of chymosin found in rennet). The temperatures for maximum milk clotting activity of kiwi and melon proteases are 40 °C and 70 °C respectively.
Enzymes are catalysts, the tireless workers responsible for building the gel. But it is casein, a group of proteins found in milk, which is the actual building block of the gel. The casein proteins group together to form large balls known as micelles which are held together by calcium ions. The outside of the micelles is covered with κ-casein which is composed of a water soluble part (an acidic glycopeptide) and another part which is much less soluble in water (para-κ-casein). The water soluble part migrates to the surface and leaves the micelles covered by a “hairy” layer. This layer both keeps the micelles dissolved in water, and prevents the micelles from coming so close to one another that they would coalesce and aggregate.
It is thanks to this “hairy” layer that milk is stable (i.e. does not spontaneously form a gel), and all is well until we add the above mentioned proteases to milk. What chymosin, GP and other proteases do is to cleave of the water soluble part of κ-casein, leaving para-κ-casein behind. Suddenly the micelles can collide, and the calcium present in milk aids in the formation of aggregates. These aggregates of “shaved” micelles make up the actual gel. It all happens within a couple of minutes. The resulting gel is very fragile and easily looses water, a process known as syneresis (see the top picture in the post).
I mentioned above the very narrow temperature window for GP, but one remaining question was whether heating milk to a higher temperature (before cooling to the same temperature window) would be beneficial. It turns out that if milk is heated above 65 °C, the strenght of the resulting gel is reduced. The reason is that the heat causes compounds in the milk (lactoglobulins) to precipitate onto the κ-casein. This interferes with the gel formation. The same is true for milk fat, so skimmed milk is the choice for a stronger gel. Since calcium plays a role in the aggregation of the “shaved” micelles, a higher calcium concentration will also result in a stronger gel.
The ginger juice also deserves a couple of extra words. In freshly squeezed ginger juice the GP has a half-life of 20 min at 30 °C, so in a warm kitchen, half of your enzyme activity is lost 20 min after you have grated and squeezed your ginger. If you leave it another 20 min you’re only left with 25% of the original activity. This means that the ginger juice can’t be prepared in advance or stored unless you use a little trick. The reason for the instability is that ginger also contains another enzyme, polyphenol oxidase (or PPO for short) – the same enzyme that is responsible for the browning of apples (an example of enzymatic browning, as opposed to the Maillard reaction which is an example of non-enzymatic browning). Once the ginger has been grated, PPO attacks phenolic groups yielding ortho-quinones. These in turn can react with the GP enzymes to inactivate them. A well-known trick to prevent the browning of apples is to use ascorbic acid (more commonly known as vitamin C). Ascorbic acid blocks the action of PPO, which in turn prevents the inactivation of the GP enzymes. And the same trick also works for ginger juice. If you need to make ginger juice in advance, just add a pinch of vitamin C (0.2% to be precise).

Ideas for further experimentation
Even though I’ve arrived at a recipe which seems to work fine, there are several claims that remain to be tested – feel free to join in on the experimentation (or share it as an idea for a school science project)!
- old vs. young ginger: I believe the ginger I can buy in Norway is not particularly young, so this is not something I’ve actually tested, but my guess is that this is far less important than temperature
- milk:ginger ratio – what is the minimum amount of ginger juice required to make the gel set?
- pour low vs. high: I believe the point of the “high pour” is to avoid any extra mixing/disturbing of the gel
- mix within the first couple of seconds: not necessary if one does a “high pour”, but mixing in the first couple of seconds should be OK, however it’s not been tested yet
- mix cold, then heat: not tested, but should work
- lemon juice contains vitamin C, so perhaps some drops of lemon juice could help stabilize the ginger juice?
- firmer gel with less sugar: not tested, but tastewise this is not relevant – and I did get a nice gel with sugar, so it’s hard to motivate this
- addition of vinegar: not tested
- add ginger juice to when the milk hits 60-65 °C: this works, but is only practical if you want to make the gel in the pan used to heat the milk, as you will probably (i.e. I have not actually tested this) not have time to pour the combined milk and ginger into a second dish; perhaps this would be relevant if making ginger curd in a microwave?
Mazorra-Manzano, M. A.; Perea-Gutiérrez, T. C.; Lugo-Sánchez, M. E.; Ramirez-Suarez, J. C.; Torres-Llanez, M.; González-Córdova, A. F.; Vallejo-Cordoba, B. “Comparison of the milk-clotting properties of three plant extracts” Food Chem. 2013, 141, 1902-1907. DOI: 10.1016/j.foodchem.2013.05.042
Su, H.-P.; Huang, M.-J.; Wang, H.-T. “Characterization of ginger proteases and their potential as a rennin replacement” J. Sci. Food Agric. 2009, 89, 1178-1185. DOI: 10.1002/jsfa.3572 [free pdf here]
Further reading
Chen, Y.-Y. “Factors Affecting Protease Activity of Ginger and Its Application in Milk Clotting Products”, 2004, Thesis (Language: Chinese).

sounds great especially if used with lactase milk, very easy digestion.
the third study mentions the results are even better than calf" rennet!
Purification, characterization, and milk coagulating properties of ginger proteases. - PubMed - NCBI
Ginger proteases are used as milk coagulants in making a Chinese traditional milk product (Jiangzhinai or Jiangzhuangnai), suggesting their potential as a source of rennet substitute that might be applicable in the modern dairy industry. In this study, ginger proteases were extracted from fresh ginger rhizome by using phosphate buffer and subsequently purified by ion exchange chromatography. Ginger proteases, all with a molecular weight around 31 kDa, were found to exist in 3 forms with isoelectric point values around 5.58, 5.40, and 5.22, respectively. These enzymes had very similar biochemical behavior, exhibiting optimal proteolytic activity from 40 to 60 °C and maximum milk clotting activity at 70 °C. They were capable of hydrolyzing isolated α(S1)-, β-, and κ-casein, of which α(S1)-casein was most susceptible to the enzyme; κ-casein was hydrolyzed with a higher specificity than α(S1)- and β-casein. In addition, the ginger proteases exhibited a similar affinity for κ-casein and higher specificity with increasing temperature. Gel electrophoresis and mass spectra indicated that Ala90-Glu91 and His102-Leu103 of κ-casein were the preferred target bonds of ginger proteases. The milk clotting activity, affinity, and specificity toward κ-casein showed that ginger protease is a promising rennet-like protease that could be used in manufacturing cheese and oriental-style dairy foods.
Characterization of ginger proteases and their potential as a rennin replacement
BACKGROUND: Ginger rhizome (Zingiber officinale Roscoe) contains ginger proteases and has proteolytic activity. Ginger proteases have been used for tenderizing meat but rarely for milk clotting. The purpose of this study was to purify ginger proteases and to research their biochemical characteristics.
RESULTS: The milk clotting activity (MCA) and proteolytic activity (PA) of the proteases was stable after storage at 4 °C for 24 h. The MCA and PA of fresh ginger juice with 0.2% L-ascorbic acid remained stable for 6 days at 4 °C. When under storage at −80 °C for 2 months, the MCA and PA of the fresh ginger juice and acetone precipitate were still high. Two peaks with protease activity were purified from a DEAE FF ion-exchange column; the specific activity (units mg−1 protein) of the MCA (MCSA) and PA (PSA) for the first peak was significantly higher than the second peak (P < 0.05). The protease activity of the ginger proteases was significantly inhibited by E-64, leupeptin, and iodoacetic acid. Zymography results showed that two protease fractions purified from ginger juice with 62 and 82 kDa had a higher PA against α- and β-casein than against κ-casein.
CONCLUSION: The ascorbic acid addition significantly stabilized the MCA and PA of ginger proteases. The protease inhibition test suggested that ginger proteases belonged to the cysteine type. The biochemical characteristics of ginger protease described in this paper can provide useful information for making new milk curd products.
Ginger protease used as coagulant enhances the proteolysis and sensory quality of Peshawari cheese compared to calf rennet
The worldwide increase in cheese consumption combined with a scarcity of rennet as well as ethical concerns have resulted in a global interest for natural milk coagulants from plant sources. In this study, the influence of ginger protease in comparison to calf rennet on the physicochemical, microbiological, and sensory characteristics of Peshawari cheese manufactured from cow’s milk was examined. For most of the physicochemical parameters (fat, protein, lactose, acidity, pH), and the main groups of microorganisms (total viable, enterobacteria, Lactobacilli, and molds and yeasts) investigated, no significant (P > 0.05) differences were observed between the two cheeses made by using different coagulants. However, significantly lower (P < 0.05) levels of moisture and higher levels of soluble nitrogen were observed in the cheese produced by ginger protease compared to that made using calf rennet. The main sensory attributes (appearance, body texture, and flavor) were significantly enhanced (P < 0.05) in Peshawari cheese prepared with ginger protease. Importantly, no bitterness was noted by the sensory panel in the Peshawari cheese made with ginger protease. The results reveal that the ginger protease may have potential application for the manufacture of Peshawari cheese.
------------
its very interesting one of those studies i posted above mentions in high temps the enzymes still active.i was under the impression that the enzyme deactivated and destroyed in higher than 45c...
Last edited:
