
New A.L.S. Treatment Lacks Evidence of Benefit, F.D.A. Panel Finds (Published 2022)
With a 6-4 vote, the group of independent advisers to the agency narrowly concluded that results from another clinical trial are needed to assess whether the therapy, called AMX0035, can help patients.
The 10-member advisory panel to the Food and Drug Administration voted 6 to 4 that available scientific data on the therapy, a two-drug combination conceived by two college students, was insufficient to show it could help patients by slowing the progression of A.L.S., a ruthless disease that impairs people’s ability to move, speak, eat and ultimately breathe.
The nonbinding vote on the therapy, AMX0035, supported the F.D.A.’s own analysis. The agency will decide whether to approve the drug in the coming months.
Groups representing patients have waged an impassioned campaign for approval, given the dearth of medications for A.L.S., also called Lou Gehrig’s disease, which often causes death within two to five years.
“There’s no question of the burdensome nature of the disease and a huge unmet need for safe and effective treatment,” said one panel member, Dr. Kenneth Fischbeck, an investigator with the National Institute of Neurological Disorders and Stroke. “On the other hand,” he said, “we were asked to look for substantial evidence with persuasiveness and robustness. And I think this one trial doesn’t quite meet that bar.”
The panel’s patient representative, Mark Weston, who has A.L.S., voted that the treatment was effective. So did Dr. Avindra Nath, a clinical director of the National Institute of Neurological Disorders and Stroke, who said, “This was a very difficult decision for me and I could have gone either way. But after weighing all the factors and facts presented I hedged over to the yes side.”
All the panelists said they were moved by fervent testimony from people living with A.L.S.
Vance Burghard testified that after being diagnosed in 2017, “I needed assistance to pull up my pants,” sometimes needed a wheelchair and “had to have my food cut for me.” A clinical trial participant, he said, “AMX0035 for me is a lifesaving and life-changing drug,” crediting it with allowing him to travel with his wife, walking “many miles in Europe, at the Great Wall of China and descending the stairs at the Potala Palace in Tibet.”
Becky Mourey, 58, was one of several patients who had lost so much speaking ability that family members read some of their words. In testimony read by her daughter, she likened A.L.S. to “those inflatable punching bags many of us had as kids — one stroke, it would bounce back up just to be hit again and again and again.”
Finishing in her own voice, Ms. Mourey, who said she has been taking the two drugs that comprise the therapy on her own, testified: “I implore you with literal life and death urgency to recommend approval of AMX0035.”
Amylyx’s co-founders, Justin Klee and Joshua Cohen, were students at Brown University less than a decade ago when they proposed that a combination of two existing drugs, taurursodiol, a supplement sometimes used to regulate liver enzymes, and sodium phenylbutyrate, a medication for a pediatric urea disorder, could safeguard neurons by preventing dysfunction of two structures in cells: mitochondria and the endoplasmic reticulum.
Sodium phenylbutyrate/ursodoxicoltaurine - Wikipedia
AMX0035 acts by blocking cell death pathways in mitochondria and in the endoplasmic reticulum.[1] Sodium phenylbutyrate is a chemical chaperone that helps proteins maintain their normal conformation, preventing aggregation that may lead to cell death.[1] Taurursodiol improves mitochondrial energy production.[1]
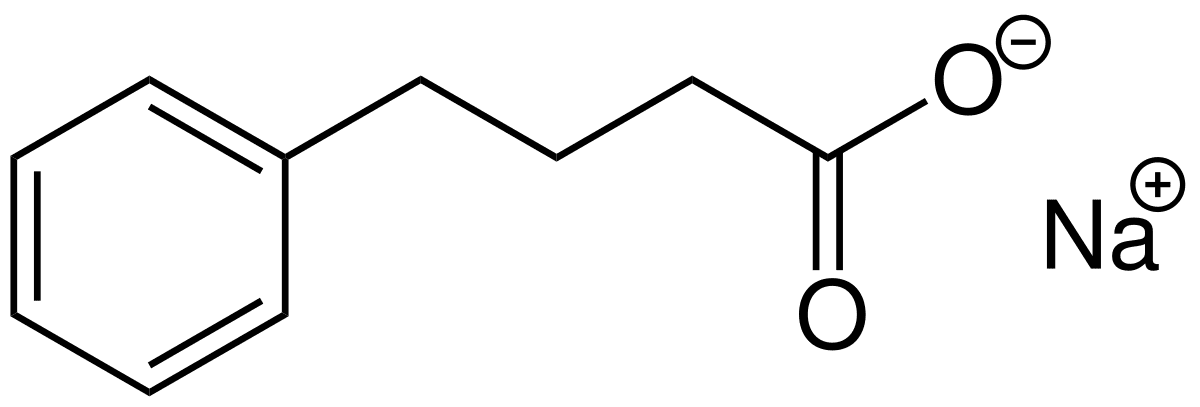
Sodium phenylbutyrate - Wikipedia
Sodium phenylbutyrate is also a histone deacetylase inhibitor and chemical chaperone, leading respectively to research into its use as an anti-cancer agent and in protein misfolding diseases such as cystic fibrosis.[2]
CYP2D6 - Wikipedia
This enzyme metabolizes several endogenous substances, such as hydroxytryptamines (Serotonin), neurosteroids, and both m-tyramine and p-tyramine which CYP2D6 metabolizes into dopamine in the brain and liver.[3][4]
