md_a
Member
- Joined
- Aug 31, 2015
- Messages
- 468
Early Trauma Influences Metabolism Across Generations
Summary: Early childhood trauma has an impact on glucose metabolism and blood composition, which are passed on to the next generation.
Source: University of Zurich
People who live through traumatic experiences in childhood often suffer long-lasting consequences that affect their mental and physical health. But moreover, their children and grand-children can also be impacted as well. In this particular form of inheritance, sperm and egg cells pass on information to offspring not through their DNA sequence like classical genetic heredity, but rather via biological factors involving the epigenome that regulates genome activity. However, the big question is how the signals triggered by traumatic events become embedded in germ cells.
“Our hypothesis was that circulating factors in blood play a role,” says Isabelle Mansuy, professor of neuroepigenetics at the University of Zurich’s Brain Research Institute and the ETH Zurich’s Institute for Neuroscience. Mansuy and her team demonstrated that childhood trauma does have a lifelong influence on blood composition and that these changes are also passed to the next generation. “These findings are extremely important for medicine, as this is the first time that a connection between early trauma and metabolic disorders in descendants is characterized,” explains Mansuy.
Traumatic stress leads to metabolic changes across generations
In her study, Mansuy used a mouse model for early trauma that had been developed in her lab. The model is used to study how the effects of trauma in early postnatal life on male mice are transmitted to their offspring. To determine whether these early experiences have an impact on blood composition, the researchers conducted multiple analyses and found large and significant differences between blood from adult traumatized animals and blood from normal, non-traumatized control group.
Changes in lipid metabolism were particularly striking, with certain polyunsaturated fatty acids metabolites appearing in higher concentrations in the blood of traumatized male mice. These same changes were also observed in their offspring. Even more strikingly, when the serum of traumatized males was chronically injected into non-traumatized males, their offspring also developed metabolic symptoms of trauma – providing a direct link between circulating factors and germ cells, thus confirming the hypothesis that blood delivers stress signals to the gametes.
Comparison with traumatized children
The researchers then investigated whether similar effects are present in humans. For this, they assembled a cohort of 25 children from an SOS Children’s Village in Pakistan who have lost their father and were separated from their mother, and analyzed their blood and saliva. When compared with children from normal families, the orphans showed higher level of several lipid metabolites – just like the traumatized mice.
“These children’s traumatic experiences are comparable to those in our mouse model, and their metabolism show similar changes in blood,” explains Mansuy. “This demonstrates the importance of animal research for providing us with fundamental insights into human health.” Up to one fourth of children across the world experience violence, abuse and neglect, that can lead to chronic diseases later in their life, highlighting the importance of Mansuy’s research.
Receptor interferes with gametes
Further experiments led the team to discover a molecular mechanism by which lipid metabolites can transmit signals to animals’ germ cells. PPAR, a receptor at the surface of cells, plays a key role in this process; it is activated by fatty acids and regulates gene expression and DNA structure in numerous tissues. The researchers discovered that this receptor is upregulated in the sperm of traumatized males.
Artificially activating this receptor in male mice led to lower body weight and disturbances in glucose metabolism – an effect that was also seen in their offspring and grand-offspring. These and other experiments led researchers to conclude that PPAR activation in sperm cells plays a significant role in the heritability of metabolic dysfunctions caused by traumatic experiences in ancestors.
Trauma damages the health of offspring
“Our findings demonstrate that early trauma influences both mental and physical health in adulthood and across generations, which can be seen in factors like lipid metabolism and glucose levels,” says Mansuy. “This is rarely taken into consideration in clinical settings.” Improving the understanding of the underlying biological processes could help medical practitioners prevent the late-onset consequences of adverse life experiences in their patients in the future.
Abstract
Involvement of circulating factors in the transmission of paternal experiences through the germline
Environmental factors can change phenotypes in exposed individuals and offspring and involve the germline, likely via biological signals in the periphery that communicate with germ cells. Here, using a mouse model of paternal exposure to traumatic stress, we identify circulating factors involving peroxisome proliferator‐activated receptor (PPAR) pathways in the effects of exposure to the germline. We show that exposure alters metabolic functions and pathways, particularly lipid‐derived metabolites, in exposed fathers and their offspring. We collected data in a human cohort exposed to childhood trauma and observed similar metabolic alterations in circulation, suggesting conserved effects. Chronic injection of serum from trauma‐exposed males into controls recapitulates metabolic phenotypes in the offspring. We identify lipid‐activated nuclear receptors PPARs as potential mediators of the effects from father to offspring. Pharmacological PPAR activation in vivo reproduces metabolic dysfunctions in the offspring and grand‐offspring of injected males and affects the sperm transcriptome in fathers and sons. In germ‐like cells in vitro, both serum and PPAR agonist induce PPAR activation. Together, these results highlight the role of circulating factors as potential communication vectors between the periphery and the germline.
Early Trauma Influences Metabolism Across Generations - Neuroscience News
...
Involvement of circulating factors in the transmission of paternal experiences through the germline
Abstract
Environmental factors can change phenotypes in exposed individuals and offspring and involve the germline, likely via biological signals in the periphery that communicate with germ cells. Here, using a mouse model of paternal exposure to traumatic stress, we identify circulating factors involving peroxisome proliferator‐activated receptor (PPAR) pathways in the effects of exposure to the germline. We show that exposure alters metabolic functions and pathways, particularly lipid‐derived metabolites, in exposed fathers and their offspring. We collected data in a human cohort exposed to childhood trauma and observed similar metabolic alterations in circulation, suggesting conserved effects. Chronic injection of serum from trauma‐exposed males into controls recapitulates metabolic phenotypes in the offspring. We identify lipid‐activated nuclear receptors PPARs as potential mediators of the effects from father to offspring. Pharmacological PPAR activation in vivo reproduces metabolic dysfunctions in the offspring and grand‐offspring of injected males and affects the sperm transcriptome in fathers and sons. In germ‐like cells in vitro, both serum and PPAR agonist induce PPAR activation. Together, these results highlight the role of circulating factors as potential communication vectors between the periphery and the germline.
Synopsis
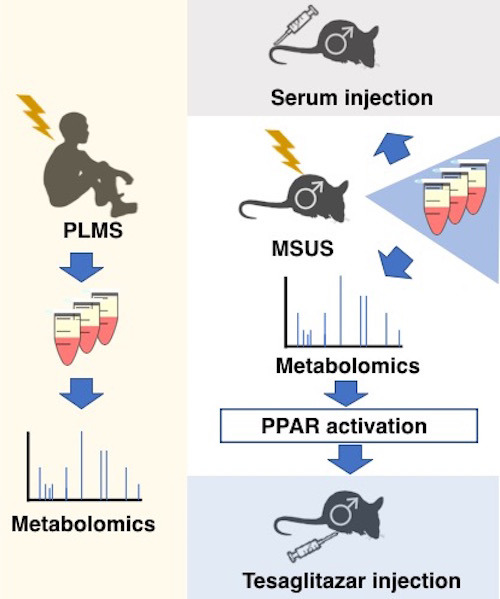
Exposure of mice to traumatic stress in early life leads to phenotypic changes that are transmitted to the progeny via mechanisms that remain poorly characterized. In vivo and in vitro findings from mice and humans implicate serum‐induced peroxisome proliferating‐activated receptor (PPAR) signaling in transmission of environmentally‐induced paternal traits via germline.
Introduction
Environmental factors and life events can have long‐lasting consequences for exposed individuals, and in some cases, they can also impact their offspring. Transmission of environmentally induced features and diseases has been overlooked for decades. But today, evidence that diet, traumatic experiences or endocrine disruptors have effects across generations has accumulated in humans and experimental animals (Bohacek & Mansuy, 2015; Nilsson et al, 2018; Panzeri & Pospisilik, 2018). These effects are known to depend on epigenetic factors and constitute an important aetiological component of many diseases. When transmitted from parent to progeny and not depending on maternal care or social factors, they are thought to involve the germline. They therefore represent a form of heredity. But how exposure can affect the germline and which signals induced by exposure in the body can reach germ cells is not known. These signals may vary depending on the type of exposure, its time window, chronicity, etc. They have in common the ability to reach germ cells. Circulating factors are important vectors of communication between tissues and cells across the body. We postulate that they can carry signals induced by exposure to germ cells and contribute to the transmission of the effects of exposure to the progeny. Blood metabolites in particular are strong candidates for being such carriers because many are potent signalling molecules, e.g. hormones, lipids, organic acids and antioxidants. Further, they are dynamically regulated by physiological states in mammals. Several metabolites have been previously implicated in the epigenetic regulation of the genome in different tissues (Donohoe & Bultman, 2012; Kaelin et al, 2013; Sharma & Rando, 2017).
Results
Blood metabolites are altered in mice exposed to early life trauma
We examined the contribution of circulating metabolites to the effects of exposure from exposed individuals to their offspring. We used an established mouse model of early postnatal trauma based on unpredictable maternal separation combined with unpredictable maternal stress (MSUS) (Fig 1A and B). Mice exposed to MSUS have metabolic dysfunctions and behavioural deficits that are transmitted to the offspring across several generations (Franklin et al, 2010; Gapp et al, 2014b, 2016b; van Steenwyk et al, 2018). We conducted unbiased metabolomic analyses in exposed adult males and their offspring using time‐of‐flight mass spectrometry (TOF‐MS). The analyses showed that polyunsaturated fatty acid (PUFA) metabolism, in particular, metabolites involved in α‐linolenic/linoleic acid (ALA/LA), and arachidonic acid (AA) pathways are significantly upregulated by MSUS in plasma of adult males (Fig 1C). PUFAs, such as eicosapentaenoic acid (EPA) and dihomo‐gamma‐linoleic acid (DGLA), and arachidonic metabolites, such as the hydroxyeicosatetraenoic acids (HETEs), were the most significantly upregulated within the enrichments (Fig 1D). In addition, bile acid biosynthesis as well as steroidogenesis and the steroidogenic ligand aldosterone were downregulated (Fig 1C, full table in Appendix Figs S1 and S2). Altered steroidogenesis is consistent with previous observation in the MSUS model that the steroid mineralocorticoid receptor (MR) is downregulated. Its pharmacological blockade mimics some MSUS effects (Gapp et al, 2014b). Remarkably, except for AA metabolism, these pathways were also altered in the offspring of MSUS males when adult (Fig 1C, Appendix Fig S1). We focused the analyses on male offspring since bodyweight is significantly affected in male but not female offspring (Appendix Fig S3).

Figure 1.Effects of early life trauma on circulating metabolites in mice and humans
We assessed the relevance of these results in humans by conducting similar analyses in a cohort of children (6‐ to 12‐year‐old girls and boys) from an SOS Children's Village in Lahore, Pakistan. The children have lost their father and were separated from their mother (paternal loss and maternal separation, PLMS) during the preceding year (Fig 1A). These conditions closely resemble the MSUS model. This human cohort is highly relevant for our study because SOS children have been exposed to a comparable trauma at a comparable age, which is key for correlative analyses with our mouse data. All SOS children live in the same orphanage, so differences in lifestyle factors are minimal. Control children were schoolmates living with both parents and not exposed to any trauma. PLMS and control groups were matched for age, body mass index and gender (Appendix Fig S4A–C). Control and PLMS groups attended the same school, with equal access to playground facilities and physical exercise. A Pakistani population was advantageous for this study because consanguinity is high in Pakistan (Bittles et al, 1991) (Appendix Fig S4D), making the group genetically more homogeneous than in other populations. Body mass index, diet and ethnicity account for < 5% of the variance between serum metabolites in healthy children from 6 different European populations (Lau et al, 2018), suggesting that our control samples are indeed comparable to other populations. Blood and saliva were collected from PLMS and control children. For these analyses and the following ones in humans, we used serum over plasma to avoid interference with clotting factors. Serum metabolites had significantly positive enrichment for AA metabolism and modest negative enrichment for bile acid biosynthesis compared to controls, with EPA, DGLA and HETEs being strongly affected (Fig 1C and D) similarly to MSUS. In saliva, both ALA/LA and AA metabolism, and steroidogenesis were also altered (Fig 1C, Appendix Fig S5), indicating alterations in different body fluids. Among the enrichments, individual metabolites in ALA/LA and AA pathways were comparably affected in MSUS and PLMS serum (Appendix Fig S6).
PPAR is activated by MSUS blood metabolites
While we consider all circulating factors altered by MSUS to be potentially involved in germline transmission, we focused on the changes in fatty acids, especially PUFA, and their metabolites. PUFAs are known to modulate metabolism, inflammation and cognitive functions. Fatty acids and their metabolites can bind to various receptors, but are particularly potent ligands for peroxisome proliferator‐activated receptors (PPARs). PPARs are widely expressed nuclear receptors that regulate gene expression and chromatin structure, and act by forming transcription factor complexes with retinoid X receptor (RXR). They can also interact with epigenetic modifying enzymes (Yu & Reddy, 2007; Romagnolo et al, 2014). Further to fatty acids, bile acids and steroid metabolites, also altered by MSUS, are ligands for nuclear receptors, in particular, farsenoid X receptor (FXR) and liver X receptor (LXR). FXR and LXR belong to the same family of nuclear receptors as PPAR and RXR, and can interact with them (Chawla et al, 2001).
Since PUFAs are PPAR ligands, we next examined if changes in circulating factors in MSUS males are linked to PPAR activity. We examined the expression of PPAR and some of their target genes in different tissues. In white adipose tissue, PPARγ is abundant and regulates adipocyte differentiation (Lee & Ge, 2014). PPARγ activation measured by transcription factor binding assay was increased in adult MSUS adipose tissue (Appendix Fig S7A). In liver, a tissue with high PPARα activity (Rakhshandehroo et al, 2010), several PPAR targets were differentially expressed, suggesting PPARα activation (Appendix Fig S7B). Further, because metabolic symptoms induced by MSUS are passed to the offspring, we also examined if germ cells have altered PPAR. PPARγ, the most abundant PPAR isotype in gametes (Aquila et al, 2006), was upregulated in MSUS sperm (Fig 2A). We then asked if metabolomic changes induced by MSUS can influence PPAR activity in germ cells. We used spermatogonial stem cell‐like cells (GC‐1 spg), diploid cells that resemble early‐stage spermatogonial cells, to assess PPAR activity in vitro. Spermatogonial cells were chosen because they are the primary germ cells present in the developing testes at the time of MSUS (first 2 weeks after birth). GC‐1 spg cells were exposed to culture medium enriched with 10% serum from control or MSUS adult males (Fig 2B). Prior to exposure, GC‐1 spg cells were transfected with a plasmid expressing luciferase under the control of a PPAR response element (PPRE). Luciferase luminescence was significantly higher in cells exposed to MSUS serum compared to control serum (Fig 2C), indicating increased PPAR activation by MSUS serum.

Figure 2.Analyses of PPAR expression and PPAR activity in sperm and germ‐like GC‐1 cells
Although previous studies have implicated PPAR and other nuclear receptors in the effects of environmental exposure and phenotype transmission (Lillycrop et al, 2008; Carone et al, 2010; Zeybel et al, 2012; Martínez et al, 2014; Baptissart et al, 2018), none have tested their causal involvement. We examined if PPAR can induce phenotype transmission by mimicking its activation in adult control males via chronic intraperitoneal (i.p.) injection of the dual PPARα/γ agonist tesaglitazar (10 μg/kg) (Fig 3A). We chose a dual agonist to activate multiple PPAR and better mimic the effects of MSUS. Following a 46‐day delay after the last injection to allow a full spermatogenesis cycle and eliminate transient effects of the drug, males were bred with control females to generate offspring. When adult, the offspring were bred with naïve females to produce grand‐offspring. Both offspring and grand‐offspring of tesaglitazar‐injected males had significantly reduced body weight compared to the offspring and grand‐offspring of vehicle‐injected controls (Fig 3B; Appendix Fig S8A), despite an initial increase at PND8 (Appendix Fig S9). This effect was not due to a difference in fathers’ weight after the injections or at the time of breeding (Appendix Fig S10). Further, blood glucose during a glucose tolerance test (GTT) was reduced in offspring and grand‐offspring compared to controls (Fig 3C; Appendix Fig S8B), suggesting increased insulin sensitivity as previously observed in MSUS offspring (Gapp et al, 2014a). However, glucose response during a restraint stress was not altered, unlike in MSUS animals (Gapp et al, 2014a; Appendix Fig S11A). This may be because tesaglitazar does not involve the stress response unlike MSUS. These results indicate that tesaglitazar can mimic some of the metabolic effects of MSUS across generations.
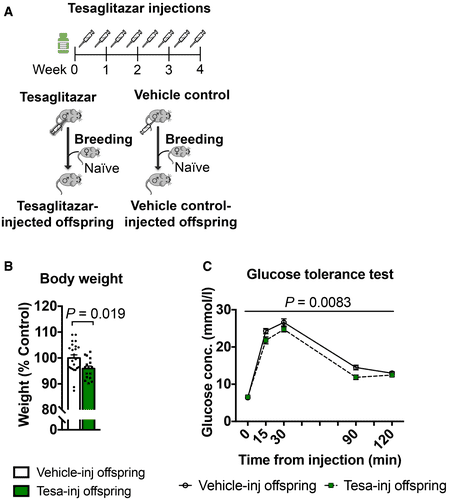
Figure 3.Tesaglitazar injection reproduces MSUS phenotypes in the offspring
MSUS‐induced shift in sperm payload is reflected in sperm of tesaglitazar‐injected fathers
Since sperm RNA has been causally involved in the transmission of the effects of MSUS to the offspring (Gapp et al, 2014a, 2020), we examined if RNA is altered in sperm of tesaglitazar‐injected males. Deep sequencing revealed differential RNA expression in sperm of tesaglitazar‐injected males compared to vehicle‐injected controls, in particular dysregulation of transposable elements (TEs) (Appendix Fig S13A). These results are consistent with previous observations in liver of tesaglitazar‐treated mice (Ferguson et al, 2018) and in sperm of males exposed to MSUS (Gapp et al, 2020). In a targeted analysis, 630 TE and > 4,000 mRNA/lincRNA transcripts were annotated and there was a significant correlation in fold change of differentially expressed TEs (MSUS versus controls and tesaglitazar versus controls), including several long terminal repeat elements (LTRs) between tesaglitazar‐injected and MSUS sperm (Fig 4A; Appendix Fig S13B). A modest fold change correlation of mRNAs/lincRNAs was also noted, in particular with lincRNAs that are the most enriched across datasets (Fig 4B; Appendix Fig S13C). Several mRNAs were also altered in sperm of tesaglitazar‐injected males (FDR < 0.05), for instance, genes involving the mitochondrial respiratory chain complex (Fig 4C and D; Appendix Fig S14C), consistent with a role for PPAR in mitochondrial metabolism (Zhang et al, 2015). Further analysis of RNA in tesaglitazar‐injected sperm confirmed significant GO term enrichments for fatty acid and lipid biosynthetic and metabolic processes (Fig 4E), pathways relevant for PPAR activity. Together, these data suggest a link between PPAR pathways in the periphery and long‐term effects on sperm RNA.

Figure 4.Differential RNA payloads in sperm from tesaglitazar‐injected males and overlap with sperm from MSUS males
RNA in sperm is thought to originate in part from earlier stages of spermatogenesis (Gapp et al, 2020), since mature sperm cells themselves are transcriptionally silent. Interestingly, sperm long RNA was not altered by tesaglitazar 1 day after the final injection unlike 46 days following the last injection (Appendix Fig S15A–D). This suggests that sperm cells are not directly affected (at least not at the level of RNA) and thus that the drug may induce changes at earlier spermatogenic stages that only appear later in mature sperm. To confirm this hypothesis, we assayed PPAR activity in GC‐1 spg cells carrying a PPRE reporter exposed to serum collected 1 day after tesaglitazar injection and observed increased luminescence compared to cells exposed to serum from vehicle‐injected controls (Appendix Fig S16). These results indicate that serum from both MSUS and tesaglitazar‐injected males can upregulate PPAR activity directly in early‐stage spermatogenic cells. Indirect effects of PPAR activation mediated by secondary factors present in circulation may, however, also occur in vivo. But this is unlikely the case with tesaglitazar since circulating metabolites are not affected by drug treatment, suggesting that there are no secondary factors at the time of breeding (Appendix Fig S17).
In vivo serum transfer induces phenotype transmission to offspring
We next tested if serum can also have effects in vivo. Blood was collected from 4‐month‐old MSUS and control males, and serum was prepared and chronically injected intravenously (i.v.) in control adult males (Fig 5A). We chose serum over plasma for in vivo injections to avoid potential interference of clotting factors. Following 4‐week treatment, males were bred with control females to generate offspring that were phenotyped when adult. The offspring of males injected with MSUS serum trended towards reduced weight (Fig 5B) and had significantly decreased blood glucose upon acute stress (Fig 5D), similar to that observed in MSUS offspring (Fig 5B and C, Gapp et al, 2014a). There was no difference in blood glucose on GTT in these offspring (Appendix Fig S11B), like in MSUS mice (Gapp et al, 2014a). The results indicate that the metabolic effects of MSUS serum are different from those of tesaglitazar treatment, which is expected since MSUS is a complex paradigm that activates components of the stress response, such as hormones, non‐lipid metabolites and other circulating and cellular constituents. To assess whether other factors than metabolites are involved, we examined proteins in plasma using unbiased mass spectrometry. No significant difference passing multiple testing corrections could be detected (Appendix Fig S18A). One interesting candidate was C‐reactive protein (CRP), which was downregulated in the proteomic datasets and could be confirmed by ELISA in a separate batch of MSUS samples (Appendix Fig S18B). CRP is a marker of inflammation linked to PPAR signalling and can be negatively regulated by PPAR activity (Zambon et al, 2006). We also examined RNA in serum since circulating miRNAs are known to communicate with tissues outside their site of origin (Thomou et al, 2017), and because RNA itself has been implicated in epigenetic inheritance (Rassoulzadegan et al, 2006; Gapp et al, 2014a; Grandjean et al, 2016). No significant difference in small RNAs could be detected in MSUS serum after multiple testing correction (Appendix Fig S19). These results suggest that circulating miRNAs probably do not play a major role, although exosomal or HDL‐associated RNA uptake (Cossetti et al, 2014) cannot be excluded. It should be noted that individual miRNAs were previously found by qPCR to be altered in MSUS serum (Gapp et al, 2014a) but this discrepancy may be due to technical differences in serum preparation or in RNA detection between RNA sequencing and qPCR.

Figure 5.Injection of serum from MSUS mice recapitulates some MSUS metabolic phenotypes in the offspring
Germ cells are the carrier of biological heredity that passes information from parent to progeny, information that is now recognized to involve both the genome and the epigenome (Chen et al, 2016). Because germ cells are sensitive to environmental factors, especially in early life (Day et al, 2016), they are subjected to alterations by exposure. If these alterations persist and are present at the time of conception, they may be transferred to the offspring. Our results (Appendix Fig S20) newly identify circulating factors as causal mediators of metabolic effects of postnatal trauma from exposed father to the offspring and highlight PPAR as one of the molecular contributors. While stress activates the sympathetic nervous system and the hypothalamic–pituitary–adrenal (HPA) axis, our results show that lipid metabolism also plays a role in the response of the body to stress exposure, and implicate PPAR beyond dietary insults (Carone et al, 2010; Zeybel et al, 2012; Chamorro‐Garcia et al, 2017). This may have important implications for the clinic since metabolic syndrome is a common co‐morbidity in the sequela of childhood trauma (Suglia et al, 2018). The results also provide a novel link between PPAR and germ cells, and a possible biological role for the known property of transcription factors to poise transcriptional states in gametes and influence the developmental trajectory of zygotes (Jung et al, 2017). The additional correlation between PPAR activation and MSUS‐induced TE dysregulation in sperm further points towards potential consequences on epigenetic control of TE‐coregulated genes in the embryo (Sharma et al, 2016). PPAR pathways in germ cells may thus contribute to differential gene expression previously observed in MSUS offspring at zygotic stage (Gapp et al, 2020). Serum factors may also act in part via somatic cells in gonads such as Sertoli, Leydig or epididymal cells which have direct contact with germ cells (Sæther et al, 2007; Sharma et al, 2016). Although the contribution of such intermediate cellular signals between circulation and germ cells cannot be ruled out, our results indicate that factors in serum are sufficient to induce the transmission of phenotypes to the offspring. The contribution of extracellular vesicles to the distribution of altered metabolites or additional factors such as RNA relevant for transmission has not been assessed but could present another potential vector for information transfer. Other mechanisms involving epigenetic processes, already implicated in phenotype transmission in several models including MSUS (Franklin et al, 2010; Gapp et al, 2014b), may also operate. It would be interesting in the future to conduct metabolomic profiling in other inter‐ or transgenerational models and in other body fluids such as lymph or seminal fluid (Di Venere et al, 2018) to clarify the role of such pathways in transmission. Moderate alterations in single metabolites are unlikely to induce transmission of the observed phenotypes on their own, yet it might be interesting to evaluate whether a large excess of individual metabolites and activation of target receptors could induce a phenotype in the offspring. Notably, our results suggest beneficial effects of tesaglitazar on offspring metabolism as reported earlier (Wallenius et al, 2013), which is consistent with the known therapeutic benefit of PUFA supplementation (Huber et al, 2007; Braarud et al, 2018). The timing and duration of PPAR activation may also be further optimized to elicit differential effects.
Finally, these findings bring new fundamental knowledge about the influence of the environment on the germline by extending the notion of non‐DNA based inheritance, known to involve epigenetic processes, to circulating factors, with important implications for heredity and evolution.
https://www.embopress.org/doi/full/10.15252/embj.2020104579
Summary: Early childhood trauma has an impact on glucose metabolism and blood composition, which are passed on to the next generation.
Source: University of Zurich
People who live through traumatic experiences in childhood often suffer long-lasting consequences that affect their mental and physical health. But moreover, their children and grand-children can also be impacted as well. In this particular form of inheritance, sperm and egg cells pass on information to offspring not through their DNA sequence like classical genetic heredity, but rather via biological factors involving the epigenome that regulates genome activity. However, the big question is how the signals triggered by traumatic events become embedded in germ cells.
“Our hypothesis was that circulating factors in blood play a role,” says Isabelle Mansuy, professor of neuroepigenetics at the University of Zurich’s Brain Research Institute and the ETH Zurich’s Institute for Neuroscience. Mansuy and her team demonstrated that childhood trauma does have a lifelong influence on blood composition and that these changes are also passed to the next generation. “These findings are extremely important for medicine, as this is the first time that a connection between early trauma and metabolic disorders in descendants is characterized,” explains Mansuy.
Traumatic stress leads to metabolic changes across generations
In her study, Mansuy used a mouse model for early trauma that had been developed in her lab. The model is used to study how the effects of trauma in early postnatal life on male mice are transmitted to their offspring. To determine whether these early experiences have an impact on blood composition, the researchers conducted multiple analyses and found large and significant differences between blood from adult traumatized animals and blood from normal, non-traumatized control group.
Changes in lipid metabolism were particularly striking, with certain polyunsaturated fatty acids metabolites appearing in higher concentrations in the blood of traumatized male mice. These same changes were also observed in their offspring. Even more strikingly, when the serum of traumatized males was chronically injected into non-traumatized males, their offspring also developed metabolic symptoms of trauma – providing a direct link between circulating factors and germ cells, thus confirming the hypothesis that blood delivers stress signals to the gametes.
Comparison with traumatized children
The researchers then investigated whether similar effects are present in humans. For this, they assembled a cohort of 25 children from an SOS Children’s Village in Pakistan who have lost their father and were separated from their mother, and analyzed their blood and saliva. When compared with children from normal families, the orphans showed higher level of several lipid metabolites – just like the traumatized mice.
“These children’s traumatic experiences are comparable to those in our mouse model, and their metabolism show similar changes in blood,” explains Mansuy. “This demonstrates the importance of animal research for providing us with fundamental insights into human health.” Up to one fourth of children across the world experience violence, abuse and neglect, that can lead to chronic diseases later in their life, highlighting the importance of Mansuy’s research.
Receptor interferes with gametes
Further experiments led the team to discover a molecular mechanism by which lipid metabolites can transmit signals to animals’ germ cells. PPAR, a receptor at the surface of cells, plays a key role in this process; it is activated by fatty acids and regulates gene expression and DNA structure in numerous tissues. The researchers discovered that this receptor is upregulated in the sperm of traumatized males.
Artificially activating this receptor in male mice led to lower body weight and disturbances in glucose metabolism – an effect that was also seen in their offspring and grand-offspring. These and other experiments led researchers to conclude that PPAR activation in sperm cells plays a significant role in the heritability of metabolic dysfunctions caused by traumatic experiences in ancestors.
Trauma damages the health of offspring
“Our findings demonstrate that early trauma influences both mental and physical health in adulthood and across generations, which can be seen in factors like lipid metabolism and glucose levels,” says Mansuy. “This is rarely taken into consideration in clinical settings.” Improving the understanding of the underlying biological processes could help medical practitioners prevent the late-onset consequences of adverse life experiences in their patients in the future.
Abstract
Involvement of circulating factors in the transmission of paternal experiences through the germline
Environmental factors can change phenotypes in exposed individuals and offspring and involve the germline, likely via biological signals in the periphery that communicate with germ cells. Here, using a mouse model of paternal exposure to traumatic stress, we identify circulating factors involving peroxisome proliferator‐activated receptor (PPAR) pathways in the effects of exposure to the germline. We show that exposure alters metabolic functions and pathways, particularly lipid‐derived metabolites, in exposed fathers and their offspring. We collected data in a human cohort exposed to childhood trauma and observed similar metabolic alterations in circulation, suggesting conserved effects. Chronic injection of serum from trauma‐exposed males into controls recapitulates metabolic phenotypes in the offspring. We identify lipid‐activated nuclear receptors PPARs as potential mediators of the effects from father to offspring. Pharmacological PPAR activation in vivo reproduces metabolic dysfunctions in the offspring and grand‐offspring of injected males and affects the sperm transcriptome in fathers and sons. In germ‐like cells in vitro, both serum and PPAR agonist induce PPAR activation. Together, these results highlight the role of circulating factors as potential communication vectors between the periphery and the germline.
Early Trauma Influences Metabolism Across Generations - Neuroscience News
...
Involvement of circulating factors in the transmission of paternal experiences through the germline
Abstract
Environmental factors can change phenotypes in exposed individuals and offspring and involve the germline, likely via biological signals in the periphery that communicate with germ cells. Here, using a mouse model of paternal exposure to traumatic stress, we identify circulating factors involving peroxisome proliferator‐activated receptor (PPAR) pathways in the effects of exposure to the germline. We show that exposure alters metabolic functions and pathways, particularly lipid‐derived metabolites, in exposed fathers and their offspring. We collected data in a human cohort exposed to childhood trauma and observed similar metabolic alterations in circulation, suggesting conserved effects. Chronic injection of serum from trauma‐exposed males into controls recapitulates metabolic phenotypes in the offspring. We identify lipid‐activated nuclear receptors PPARs as potential mediators of the effects from father to offspring. Pharmacological PPAR activation in vivo reproduces metabolic dysfunctions in the offspring and grand‐offspring of injected males and affects the sperm transcriptome in fathers and sons. In germ‐like cells in vitro, both serum and PPAR agonist induce PPAR activation. Together, these results highlight the role of circulating factors as potential communication vectors between the periphery and the germline.
Synopsis

Exposure of mice to traumatic stress in early life leads to phenotypic changes that are transmitted to the progeny via mechanisms that remain poorly characterized. In vivo and in vitro findings from mice and humans implicate serum‐induced peroxisome proliferating‐activated receptor (PPAR) signaling in transmission of environmentally‐induced paternal traits via germline.
- Lipid metabolism pathways are altered in plasma of adult male mice exposed to postnatal trauma and their adult offspring
- Lipid metabolism is also altered in plasma and saliva of human orphan children.
- Serum from mice with postnatal trauma activates PPAR nuclear receptors in spermatogonial cells in vitro.
- Pharmacological PPAR activation in vivo recapitulates trauma‐induced metabolic phenotypes in adult mice and their offspring and sperm RNA alterations in fathers.
- Injection of serum from trauma‐exposed males into control males recapitulates metabolic symptoms in their offspring.
Introduction
Environmental factors and life events can have long‐lasting consequences for exposed individuals, and in some cases, they can also impact their offspring. Transmission of environmentally induced features and diseases has been overlooked for decades. But today, evidence that diet, traumatic experiences or endocrine disruptors have effects across generations has accumulated in humans and experimental animals (Bohacek & Mansuy, 2015; Nilsson et al, 2018; Panzeri & Pospisilik, 2018). These effects are known to depend on epigenetic factors and constitute an important aetiological component of many diseases. When transmitted from parent to progeny and not depending on maternal care or social factors, they are thought to involve the germline. They therefore represent a form of heredity. But how exposure can affect the germline and which signals induced by exposure in the body can reach germ cells is not known. These signals may vary depending on the type of exposure, its time window, chronicity, etc. They have in common the ability to reach germ cells. Circulating factors are important vectors of communication between tissues and cells across the body. We postulate that they can carry signals induced by exposure to germ cells and contribute to the transmission of the effects of exposure to the progeny. Blood metabolites in particular are strong candidates for being such carriers because many are potent signalling molecules, e.g. hormones, lipids, organic acids and antioxidants. Further, they are dynamically regulated by physiological states in mammals. Several metabolites have been previously implicated in the epigenetic regulation of the genome in different tissues (Donohoe & Bultman, 2012; Kaelin et al, 2013; Sharma & Rando, 2017).
Results
Blood metabolites are altered in mice exposed to early life trauma
We examined the contribution of circulating metabolites to the effects of exposure from exposed individuals to their offspring. We used an established mouse model of early postnatal trauma based on unpredictable maternal separation combined with unpredictable maternal stress (MSUS) (Fig 1A and B). Mice exposed to MSUS have metabolic dysfunctions and behavioural deficits that are transmitted to the offspring across several generations (Franklin et al, 2010; Gapp et al, 2014b, 2016b; van Steenwyk et al, 2018). We conducted unbiased metabolomic analyses in exposed adult males and their offspring using time‐of‐flight mass spectrometry (TOF‐MS). The analyses showed that polyunsaturated fatty acid (PUFA) metabolism, in particular, metabolites involved in α‐linolenic/linoleic acid (ALA/LA), and arachidonic acid (AA) pathways are significantly upregulated by MSUS in plasma of adult males (Fig 1C). PUFAs, such as eicosapentaenoic acid (EPA) and dihomo‐gamma‐linoleic acid (DGLA), and arachidonic metabolites, such as the hydroxyeicosatetraenoic acids (HETEs), were the most significantly upregulated within the enrichments (Fig 1D). In addition, bile acid biosynthesis as well as steroidogenesis and the steroidogenic ligand aldosterone were downregulated (Fig 1C, full table in Appendix Figs S1 and S2). Altered steroidogenesis is consistent with previous observation in the MSUS model that the steroid mineralocorticoid receptor (MR) is downregulated. Its pharmacological blockade mimics some MSUS effects (Gapp et al, 2014b). Remarkably, except for AA metabolism, these pathways were also altered in the offspring of MSUS males when adult (Fig 1C, Appendix Fig S1). We focused the analyses on male offspring since bodyweight is significantly affected in male but not female offspring (Appendix Fig S3).

Figure 1.Effects of early life trauma on circulating metabolites in mice and humans
- Paradigms of early life trauma in mice and humans. In mice, early life trauma consists of an exposure to unpredictable maternal separation combined with unpredictable maternal stress (MSUS). In humans, exposure involves paternal loss and maternal separation (PLMS) in early childhood (age 6–12).
- Scheme illustrating the MSUS model and control mice showing blood collection and breeding to generate an offspring. For MSUS (symbolized by yellow blitz), newborn pups are separated from their mother unpredictably 3 h/day from postnatal day (PND) 1–14. During separation, the dam is exposed to different stressors unpredictably9. Serum was prepared from blood collected from 3‐month‐old MSUS and control males.
- Differential pathway enrichment of metabolites in MSUS plasma from adult males and their offspring compared to controls (each group n = 5), and serum (PLMS, n = 20; control, n = 14) and saliva (PLMS, n = 25; control, n = 14) from PLMS and control children. Asterisk and hashtag represent FDR after multiple testing corrections using Benjamini–Hochberg (BH) test. Columns indicate significance for positive (+) and negative (−) enrichment.
- Individual metabolites in ALA/LA and AA pathways significantly altered in both MSUS and PLMS. Numbers represent fold change according to a heat scale (right).
We assessed the relevance of these results in humans by conducting similar analyses in a cohort of children (6‐ to 12‐year‐old girls and boys) from an SOS Children's Village in Lahore, Pakistan. The children have lost their father and were separated from their mother (paternal loss and maternal separation, PLMS) during the preceding year (Fig 1A). These conditions closely resemble the MSUS model. This human cohort is highly relevant for our study because SOS children have been exposed to a comparable trauma at a comparable age, which is key for correlative analyses with our mouse data. All SOS children live in the same orphanage, so differences in lifestyle factors are minimal. Control children were schoolmates living with both parents and not exposed to any trauma. PLMS and control groups were matched for age, body mass index and gender (Appendix Fig S4A–C). Control and PLMS groups attended the same school, with equal access to playground facilities and physical exercise. A Pakistani population was advantageous for this study because consanguinity is high in Pakistan (Bittles et al, 1991) (Appendix Fig S4D), making the group genetically more homogeneous than in other populations. Body mass index, diet and ethnicity account for < 5% of the variance between serum metabolites in healthy children from 6 different European populations (Lau et al, 2018), suggesting that our control samples are indeed comparable to other populations. Blood and saliva were collected from PLMS and control children. For these analyses and the following ones in humans, we used serum over plasma to avoid interference with clotting factors. Serum metabolites had significantly positive enrichment for AA metabolism and modest negative enrichment for bile acid biosynthesis compared to controls, with EPA, DGLA and HETEs being strongly affected (Fig 1C and D) similarly to MSUS. In saliva, both ALA/LA and AA metabolism, and steroidogenesis were also altered (Fig 1C, Appendix Fig S5), indicating alterations in different body fluids. Among the enrichments, individual metabolites in ALA/LA and AA pathways were comparably affected in MSUS and PLMS serum (Appendix Fig S6).
PPAR is activated by MSUS blood metabolites
While we consider all circulating factors altered by MSUS to be potentially involved in germline transmission, we focused on the changes in fatty acids, especially PUFA, and their metabolites. PUFAs are known to modulate metabolism, inflammation and cognitive functions. Fatty acids and their metabolites can bind to various receptors, but are particularly potent ligands for peroxisome proliferator‐activated receptors (PPARs). PPARs are widely expressed nuclear receptors that regulate gene expression and chromatin structure, and act by forming transcription factor complexes with retinoid X receptor (RXR). They can also interact with epigenetic modifying enzymes (Yu & Reddy, 2007; Romagnolo et al, 2014). Further to fatty acids, bile acids and steroid metabolites, also altered by MSUS, are ligands for nuclear receptors, in particular, farsenoid X receptor (FXR) and liver X receptor (LXR). FXR and LXR belong to the same family of nuclear receptors as PPAR and RXR, and can interact with them (Chawla et al, 2001).
Since PUFAs are PPAR ligands, we next examined if changes in circulating factors in MSUS males are linked to PPAR activity. We examined the expression of PPAR and some of their target genes in different tissues. In white adipose tissue, PPARγ is abundant and regulates adipocyte differentiation (Lee & Ge, 2014). PPARγ activation measured by transcription factor binding assay was increased in adult MSUS adipose tissue (Appendix Fig S7A). In liver, a tissue with high PPARα activity (Rakhshandehroo et al, 2010), several PPAR targets were differentially expressed, suggesting PPARα activation (Appendix Fig S7B). Further, because metabolic symptoms induced by MSUS are passed to the offspring, we also examined if germ cells have altered PPAR. PPARγ, the most abundant PPAR isotype in gametes (Aquila et al, 2006), was upregulated in MSUS sperm (Fig 2A). We then asked if metabolomic changes induced by MSUS can influence PPAR activity in germ cells. We used spermatogonial stem cell‐like cells (GC‐1 spg), diploid cells that resemble early‐stage spermatogonial cells, to assess PPAR activity in vitro. Spermatogonial cells were chosen because they are the primary germ cells present in the developing testes at the time of MSUS (first 2 weeks after birth). GC‐1 spg cells were exposed to culture medium enriched with 10% serum from control or MSUS adult males (Fig 2B). Prior to exposure, GC‐1 spg cells were transfected with a plasmid expressing luciferase under the control of a PPAR response element (PPRE). Luciferase luminescence was significantly higher in cells exposed to MSUS serum compared to control serum (Fig 2C), indicating increased PPAR activation by MSUS serum.

Figure 2.Analyses of PPAR expression and PPAR activity in sperm and germ‐like GC‐1 cells
- PPARγ mRNA expression in sperm from control and MSUS mice. Control, n = 15; MSUS, n = 13, two‐tailed Student's t‐test, P = 0.029, t = 2.30, df = 26.
- Schematic of serum treatment of GC‐1 cells. On day 1, cells are transfected with PPRE and luciferase normalization plasmids. Serum is mixed in culture medium at 10% concentration and exposed to cells on day 2. Cells are harvested 24 h later (Day 3), and luminescence is measured.
- Relative luciferase luminescence in transfected GC‐1 cells exposed to serum from control or MSUS mice. Numbers correspond to serum from individual animals applied to different cell culture wells. Control, n = 17; MSUS, n = 22, two‐tailed Student's t‐test, P = 0.042, t = 2.1, df = 37.
Although previous studies have implicated PPAR and other nuclear receptors in the effects of environmental exposure and phenotype transmission (Lillycrop et al, 2008; Carone et al, 2010; Zeybel et al, 2012; Martínez et al, 2014; Baptissart et al, 2018), none have tested their causal involvement. We examined if PPAR can induce phenotype transmission by mimicking its activation in adult control males via chronic intraperitoneal (i.p.) injection of the dual PPARα/γ agonist tesaglitazar (10 μg/kg) (Fig 3A). We chose a dual agonist to activate multiple PPAR and better mimic the effects of MSUS. Following a 46‐day delay after the last injection to allow a full spermatogenesis cycle and eliminate transient effects of the drug, males were bred with control females to generate offspring. When adult, the offspring were bred with naïve females to produce grand‐offspring. Both offspring and grand‐offspring of tesaglitazar‐injected males had significantly reduced body weight compared to the offspring and grand‐offspring of vehicle‐injected controls (Fig 3B; Appendix Fig S8A), despite an initial increase at PND8 (Appendix Fig S9). This effect was not due to a difference in fathers’ weight after the injections or at the time of breeding (Appendix Fig S10). Further, blood glucose during a glucose tolerance test (GTT) was reduced in offspring and grand‐offspring compared to controls (Fig 3C; Appendix Fig S8B), suggesting increased insulin sensitivity as previously observed in MSUS offspring (Gapp et al, 2014a). However, glucose response during a restraint stress was not altered, unlike in MSUS animals (Gapp et al, 2014a; Appendix Fig S11A). This may be because tesaglitazar does not involve the stress response unlike MSUS. These results indicate that tesaglitazar can mimic some of the metabolic effects of MSUS across generations.

Figure 3.Tesaglitazar injection reproduces MSUS phenotypes in the offspring
- Control males were injected with either tesaglitazar (grey syringe) or vehicle twice per week for 4 weeks. Males were paired with control females to generate offspring.
- Adult weight in the offspring of tesaglitazar‐injected males (Tesa‐inj, n = 16) compared to the offspring of vehicle‐injected males (Vehicle‐inj, n = 23). Two‐tailed Student's t‐test, P = 0.019, t = 2.44, df = 37.
- Glucose level in the offspring of Tesa‐inj (n = 14) and Vehicle‐inj (n = 21) males during a glucose tolerance test. Repeated‐measures ANOVA, treatment effect P = 0.0083, F (1, 33) = 7.877, time effect P < 0.0001, F (4, 132) = 347.7, interaction P = 0.0776, F (4, 132) = 2.155 Conc.; concentration.
MSUS‐induced shift in sperm payload is reflected in sperm of tesaglitazar‐injected fathers
Since sperm RNA has been causally involved in the transmission of the effects of MSUS to the offspring (Gapp et al, 2014a, 2020), we examined if RNA is altered in sperm of tesaglitazar‐injected males. Deep sequencing revealed differential RNA expression in sperm of tesaglitazar‐injected males compared to vehicle‐injected controls, in particular dysregulation of transposable elements (TEs) (Appendix Fig S13A). These results are consistent with previous observations in liver of tesaglitazar‐treated mice (Ferguson et al, 2018) and in sperm of males exposed to MSUS (Gapp et al, 2020). In a targeted analysis, 630 TE and > 4,000 mRNA/lincRNA transcripts were annotated and there was a significant correlation in fold change of differentially expressed TEs (MSUS versus controls and tesaglitazar versus controls), including several long terminal repeat elements (LTRs) between tesaglitazar‐injected and MSUS sperm (Fig 4A; Appendix Fig S13B). A modest fold change correlation of mRNAs/lincRNAs was also noted, in particular with lincRNAs that are the most enriched across datasets (Fig 4B; Appendix Fig S13C). Several mRNAs were also altered in sperm of tesaglitazar‐injected males (FDR < 0.05), for instance, genes involving the mitochondrial respiratory chain complex (Fig 4C and D; Appendix Fig S14C), consistent with a role for PPAR in mitochondrial metabolism (Zhang et al, 2015). Further analysis of RNA in tesaglitazar‐injected sperm confirmed significant GO term enrichments for fatty acid and lipid biosynthetic and metabolic processes (Fig 4E), pathways relevant for PPAR activity. Together, these data suggest a link between PPAR pathways in the periphery and long‐term effects on sperm RNA.

Figure 4.Differential RNA payloads in sperm from tesaglitazar‐injected males and overlap with sperm from MSUS males
- A, B.Differentially expressed (A) transposable elements and (B) mRNAs/lincRNAs in sperm from Tesa‐inj and MSUS males. Data represent genes with P < 0.05 and similar fold change. Fold change in heat map represents log2(fold change) respective to the corresponding control group. Total overlap is presented in Appendix Fig S13.
- C, D.(C) Volcano plot and (D) heat map of differentially expressed (FDR < 0.05) mRNA/lincRNA in sperm from Tesa‐inj males. Dashed black lines in (C) represent y = 1.3, equivalent to FDR = 0.05, and x = ±0.03, equivalent to FC = 1.23 and 0.81. Black dots represent top candidates, which all have FC > 2 or FC < 0.5, and FDR < 0.05. Teal dots represent non‐significant genes.
- E.GO enrichment scores for differential RNA in Tesa‐inj male sperm. Enrichments calculated with P < 0.05. For Tesa‐inj, n = 6; Vehicle‐inj, n = 7; MSUS, n = 4; and control, n = 3. Dashed black line represents FDR = 0.05. FDR, false discovery rate; FC, fold change.
RNA in sperm is thought to originate in part from earlier stages of spermatogenesis (Gapp et al, 2020), since mature sperm cells themselves are transcriptionally silent. Interestingly, sperm long RNA was not altered by tesaglitazar 1 day after the final injection unlike 46 days following the last injection (Appendix Fig S15A–D). This suggests that sperm cells are not directly affected (at least not at the level of RNA) and thus that the drug may induce changes at earlier spermatogenic stages that only appear later in mature sperm. To confirm this hypothesis, we assayed PPAR activity in GC‐1 spg cells carrying a PPRE reporter exposed to serum collected 1 day after tesaglitazar injection and observed increased luminescence compared to cells exposed to serum from vehicle‐injected controls (Appendix Fig S16). These results indicate that serum from both MSUS and tesaglitazar‐injected males can upregulate PPAR activity directly in early‐stage spermatogenic cells. Indirect effects of PPAR activation mediated by secondary factors present in circulation may, however, also occur in vivo. But this is unlikely the case with tesaglitazar since circulating metabolites are not affected by drug treatment, suggesting that there are no secondary factors at the time of breeding (Appendix Fig S17).
In vivo serum transfer induces phenotype transmission to offspring
We next tested if serum can also have effects in vivo. Blood was collected from 4‐month‐old MSUS and control males, and serum was prepared and chronically injected intravenously (i.v.) in control adult males (Fig 5A). We chose serum over plasma for in vivo injections to avoid potential interference of clotting factors. Following 4‐week treatment, males were bred with control females to generate offspring that were phenotyped when adult. The offspring of males injected with MSUS serum trended towards reduced weight (Fig 5B) and had significantly decreased blood glucose upon acute stress (Fig 5D), similar to that observed in MSUS offspring (Fig 5B and C, Gapp et al, 2014a). There was no difference in blood glucose on GTT in these offspring (Appendix Fig S11B), like in MSUS mice (Gapp et al, 2014a). The results indicate that the metabolic effects of MSUS serum are different from those of tesaglitazar treatment, which is expected since MSUS is a complex paradigm that activates components of the stress response, such as hormones, non‐lipid metabolites and other circulating and cellular constituents. To assess whether other factors than metabolites are involved, we examined proteins in plasma using unbiased mass spectrometry. No significant difference passing multiple testing corrections could be detected (Appendix Fig S18A). One interesting candidate was C‐reactive protein (CRP), which was downregulated in the proteomic datasets and could be confirmed by ELISA in a separate batch of MSUS samples (Appendix Fig S18B). CRP is a marker of inflammation linked to PPAR signalling and can be negatively regulated by PPAR activity (Zambon et al, 2006). We also examined RNA in serum since circulating miRNAs are known to communicate with tissues outside their site of origin (Thomou et al, 2017), and because RNA itself has been implicated in epigenetic inheritance (Rassoulzadegan et al, 2006; Gapp et al, 2014a; Grandjean et al, 2016). No significant difference in small RNAs could be detected in MSUS serum after multiple testing correction (Appendix Fig S19). These results suggest that circulating miRNAs probably do not play a major role, although exosomal or HDL‐associated RNA uptake (Cossetti et al, 2014) cannot be excluded. It should be noted that individual miRNAs were previously found by qPCR to be altered in MSUS serum (Gapp et al, 2014a) but this discrepancy may be due to technical differences in serum preparation or in RNA detection between RNA sequencing and qPCR.

Figure 5.Injection of serum from MSUS mice recapitulates some MSUS metabolic phenotypes in the offspring
- A.Serum was injected (90 μl) twice per week for 4 weeks into age‐matched control males. After injection, the males were paired with control females and their offspring were phenotyped when 3 months old and compared to the offspring of MSUS males.
- B.Weight in adult male MSUS offspring and the offspring of males injected with MSUS serum compared to respective control groups. MSUS offspring, n = 22; control offspring, n = 24, one‐tailed Student's t‐test (data reproduced) P = 0.045, t = 1.734, df = 44. MSUS serum‐injected offspring, n = 30; control serum‐injected offspring, n = 31, two‐tailed Mann–Whitney U = 334.5, P = 0.059.
- C, D.Blood glucose levels in MSUS offspring and the offspring of MSUS serum‐injected males following a 30‐min restraint challenge. MSUS offspring, n = 13; control offspring, n = 12, repeat‐measures ANOVA, treatment effect P = 0.016, F (1, 23) = 6.704, time effect P < 0.0001, F (3, 69) = 53.13, interaction P = 0.027, F (3, 69) = 3.25, at 15 min adjusted P = 0.0015, t = 3.693, df = 92. MSUS serum‐injected offspring, n = 17; and control serum‐injected offspring, n = 14, repeat‐measures ANOVA, treatment effect P = 0.023, F (1,14) = 6.493, time effect P < 0.0001, F (3, 42) = 48.4, interaction P = 0.29, F (3, 42) = 1.29. Conc., concentration.
Germ cells are the carrier of biological heredity that passes information from parent to progeny, information that is now recognized to involve both the genome and the epigenome (Chen et al, 2016). Because germ cells are sensitive to environmental factors, especially in early life (Day et al, 2016), they are subjected to alterations by exposure. If these alterations persist and are present at the time of conception, they may be transferred to the offspring. Our results (Appendix Fig S20) newly identify circulating factors as causal mediators of metabolic effects of postnatal trauma from exposed father to the offspring and highlight PPAR as one of the molecular contributors. While stress activates the sympathetic nervous system and the hypothalamic–pituitary–adrenal (HPA) axis, our results show that lipid metabolism also plays a role in the response of the body to stress exposure, and implicate PPAR beyond dietary insults (Carone et al, 2010; Zeybel et al, 2012; Chamorro‐Garcia et al, 2017). This may have important implications for the clinic since metabolic syndrome is a common co‐morbidity in the sequela of childhood trauma (Suglia et al, 2018). The results also provide a novel link between PPAR and germ cells, and a possible biological role for the known property of transcription factors to poise transcriptional states in gametes and influence the developmental trajectory of zygotes (Jung et al, 2017). The additional correlation between PPAR activation and MSUS‐induced TE dysregulation in sperm further points towards potential consequences on epigenetic control of TE‐coregulated genes in the embryo (Sharma et al, 2016). PPAR pathways in germ cells may thus contribute to differential gene expression previously observed in MSUS offspring at zygotic stage (Gapp et al, 2020). Serum factors may also act in part via somatic cells in gonads such as Sertoli, Leydig or epididymal cells which have direct contact with germ cells (Sæther et al, 2007; Sharma et al, 2016). Although the contribution of such intermediate cellular signals between circulation and germ cells cannot be ruled out, our results indicate that factors in serum are sufficient to induce the transmission of phenotypes to the offspring. The contribution of extracellular vesicles to the distribution of altered metabolites or additional factors such as RNA relevant for transmission has not been assessed but could present another potential vector for information transfer. Other mechanisms involving epigenetic processes, already implicated in phenotype transmission in several models including MSUS (Franklin et al, 2010; Gapp et al, 2014b), may also operate. It would be interesting in the future to conduct metabolomic profiling in other inter‐ or transgenerational models and in other body fluids such as lymph or seminal fluid (Di Venere et al, 2018) to clarify the role of such pathways in transmission. Moderate alterations in single metabolites are unlikely to induce transmission of the observed phenotypes on their own, yet it might be interesting to evaluate whether a large excess of individual metabolites and activation of target receptors could induce a phenotype in the offspring. Notably, our results suggest beneficial effects of tesaglitazar on offspring metabolism as reported earlier (Wallenius et al, 2013), which is consistent with the known therapeutic benefit of PUFA supplementation (Huber et al, 2007; Braarud et al, 2018). The timing and duration of PPAR activation may also be further optimized to elicit differential effects.
Finally, these findings bring new fundamental knowledge about the influence of the environment on the germline by extending the notion of non‐DNA based inheritance, known to involve epigenetic processes, to circulating factors, with important implications for heredity and evolution.
https://www.embopress.org/doi/full/10.15252/embj.2020104579


