Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
The diamine cadaverine is formed from the decarboxylation of lysine, and the diamine putrescine is formed upon decarboxylation of ornithine—formed from either methionine or arginine. It has been shown in the rat that ornithine decarboxylase—the main polyamine producing enzyme—is also responsible for the decarboxylation of lysine.*So would supplementing lysine probably not be a good idea?
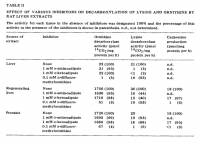
Lysine decarboxylase activity executed by ornithine decarboxylase.
'The present experiments indicate that rat tissues do contain a lysine decarboxylase activity capable of producing cadaverine, but indicate that all of this activity is due to the action of ornithine decarboxylase.' ―Pegg
'Persson [15] has also concluded that mouse kidney ornithine decarboxylase acts on lysine.' ―Pegg
'The present experiments are in agreement with those of Bey et al. [37] in indicating that the active center for omithine decarboxylase strongly prefers the distance between the amino groups to be that of four carbon atoms. However, it is shown here five carbons can be accommodated with a reduced affinity.' ―Pegg
'But this lysine decarboxylase activity by ornithine decarboxylase, to form cadaverine, is less specific and slower than the decarboxylation of ornithine to produce putrescine. Lysine is less of a substrate, and much less cadaverine is produced in tissues.' ―Pegg
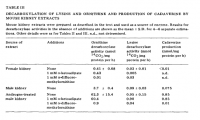
'For this reason, although the V with lysine was about 25% that with ornithine (Table V), the degree of lysine decarboxylation is likely to be small in most tissues' ―Pegg
'Persson [15] has also concluded that mouse kidney ornithine decarboxylase acts on lysine.' ―Pegg
'The present experiments are in agreement with those of Bey et al. [37] in indicating that the active center for omithine decarboxylase strongly prefers the distance between the amino groups to be that of four carbon atoms. However, it is shown here five carbons can be accommodated with a reduced affinity.' ―Pegg
'But this lysine decarboxylase activity by ornithine decarboxylase, to form cadaverine, is less specific and slower than the decarboxylation of ornithine to produce putrescine. Lysine is less of a substrate, and much less cadaverine is produced in tissues.' ―Pegg
'For this reason, although the V with lysine was about 25% that with ornithine (Table V), the degree of lysine decarboxylation is likely to be small in most tissues' ―Pegg
'However, it remains possible that in tissues other than liver which has a relatively high ornithine content, there might be significant decarboxylation of lysine to form cadaverine. In particular, the possibility that cells in culture which may be exposed to substantially higher concentrations of lysine than ornithine may produce cadaverine as well as putrescine appears to be worthy of consideration.' ―Pegg
Methionine and arginine seem of more concern, as they both form ornithine—the substrate of higher affinity. But since arginine can be disabled by methylgloxal (imidazalone formation), this amino acid could be less of a factor. (Arginine is also responsible for the body's nitric oxide.) Most researchers seem to agree that excess methionine determines polyamine synthesis more than any other amino acid.
Polyamines increase growth, and may be desired—by some—for this reason. But if a person wants a reduction in size and growth, a reduction in these aforementioned amino acids may be a good idea. If someone has cancer, the amino acid threonine would probably be the one to consume—certainly not methionine. Threonine is a precursor to methylglyoxal.
I think part of the reason why Gerson and Koch give people fruit is because it is lower in total protein than most foods, and also has a lower ratio of methionine over the total protein (and has vitamins!). Once a tumor has formed, I think it's fair to assume that it has a higher ornithine decarboxylase activity than most tissues;† tumors have been shown to turn excessive methionine into polyamines at a relatively quick rate. In cancer, it could be a good to have arginine and methione as the limiting amino acids. This can probably be accomplished by consuming fruit, leaves, and gelatine—essentially the same dietary strategy found effective by Gerson and Koch. (But Charlotte Gerson is deluding herself with linoleic acid recommendations; either that, or she's getting paid to discredit her own Dad's research (facetious). Linoleic acid is one of the main risk factors in prostate cancer; it probably does this by first becoming leukotriene B4, which then goes on the upregulate the enzyme ornithine decarboxylase (of all things).).‡
[*] Pegg, Anthony E. "Decarboxylation of ornithine and lysine in rat tissues." Biochimica et Biophysica Acta (BBA)-Enzymology (1979)
[†] O'Brien, Thomas. "Ornithine decarboxylase overexpression is a sufficient condition for tumor promotion in mouse skin." Cancer Research (1997)
[‡] Belury, Martha A. "Dietary conjugated linoleic acid induces peroxisome-specific enzyme accumulation and ornithine decarboxylase activity in mouse liver." The Journal of nutritional biochemistry (1997)
[†] O'Brien, Thomas. "Ornithine decarboxylase overexpression is a sufficient condition for tumor promotion in mouse skin." Cancer Research (1997)
[‡] Belury, Martha A. "Dietary conjugated linoleic acid induces peroxisome-specific enzyme accumulation and ornithine decarboxylase activity in mouse liver." The Journal of nutritional biochemistry (1997)
Last edited: