md_a
Member
- Joined
- Aug 31, 2015
- Messages
- 468
Increased Carbon Monoxide in Exhaled Air of Subjects with Upper Respiratory Tract Infections
Received: November 14, 1997
Viral infection may induce the expression of heme oxygenase, resulting in increased carbon monoxide (CO) formation. CO may be produced by various cells of the upper and lower respiratory tract and may be detected in the exhaled air. Therefore, exhaled CO concentrations were measured on a CO monitor by vital capacity maneuver in subjects with upper respiratory tract infections (URTIs) and in nonsmoking and smoking healthy control subjects. At the time of symptoms of URTI, exhaled CO concentrations were 5.6 ± 0.4 ppm and decreased to 1.0 ± 0.1 ppm during recovery. Recovery values of exhaled CO were similar to those in age-matched nonsmoking healthy control subjects (1.2 ± 0.3 ppm). Smoking healthy control subjects had the highest levels of exhaled CO concentration among the groups (18.5 ± 2.5 ppm). These findings suggest that symptomatic URTIs increase the concentration of CO in exhaled air. This may reflect the induction of heme oxygenase that has an antiviral effect in the airways.
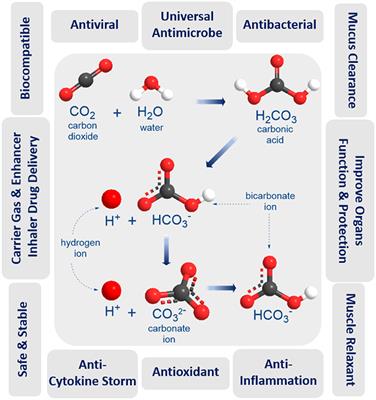
Carbon monoxide (CO) has been reported to have several biologic actions (1-4) and may play a role in pathophysiology of airway diseases (5). CO is made by an enzyme called heme oxygenase, and two forms of heme oxygenase have been characterized (6). Constitutive forms of the enzyme are widely distributed throughout the body with high concentrations in the brain (6), but another isoform is induced in several types of cells after exposure to inflammatory cytokines (7, 8), oxidants (9, 10) and NO (11). CO is detectable in the exhaled air of normal persons (5, 12) and exhaled CO is increased in asthma (5), which may reflect an expression of inducible heme oxygenase in airway epithelial cells (13). This is supported by the fact that inhaled corticosteroids inhibit the increase in exhaled CO in asthmatic patients (5).
DISCUSSION
The present study has shown that exhaled CO can be reliably measured in healthy control subjects and subjects during the acute phase of URTIs. The values of exhaled CO in nonsmoking and smoking control subjects were similar to those of previous studies (12, 21, 22). Because the carboxyhemoglobin level in the blood declines exponentially and becomes normal 24 h after the cessation of smoking (23), the present study should underestimate the exhaled CO concentration in smoking control subjects. However, we have demonstrated that URTIs are associated with an increase in exhaled CO in normal persons in the acute phase when symptoms are present, and that there is a reduction in exhaled CO after recovery to values that are similar to those in age-matched normal subjects. This suggests that URTI, presumably caused by influenza viral infection, increases the production of CO in the respiratory tract.
However, several factors might influence the exhaled CO concentration in the present study. First, some variations in the exhaled CO concentration among three sequential maneuvers were observed. Although the reason for observed variations is uncertain, the mean exhaled CO concentrations were similar among three sequential maneuvers in nonsmoking control subjects and the subjects during and after URTIs. Furthermore, the variation between the exhaled CO concentrations on separate days in nonsmoking control subjects was small. Second, the subjects exposed to cigarette smoke as passive smokers might have the same range as the subjects with URTIs. However, it seems unlikely because we selected the subjects with URTIs and none of them were smokers, ex-smokers, or passive smokers. Finally, the subjects might encounter much higher ambient CO levels than those at the place of the CO measurement on their way to the hospital. However, the route and means of transportation to the hospital were the same in the subjects with URTIs between the time of the acute phase and after 3 wk of recovery, and they visited the hospital at a similar time of day. Therefore, the ambient CO levels on the way to the hospital could little influence the difference in the exhaled CO concentration between the time of the acute phase and after 3 wk of recovery in the subjects with URTIs.
The exhaled CO concentration was higher without an expiratory resistance used to close the velum during expiration to exclude nasal CO that may leak throughout expiration in the presence of an open velum (20). Therefore, the increase in CO may be derived from both nasal tissues and the lower respiratory tract. It is of interest that a large proportion of subjects with URTIs in this study complained of lower respiratory tract symptoms, including cough and chest pain accentuated by coughing, indicating that the lower respiratory tract was likely to have been involved.
Heme oxygenase plays an important role in the resolution of inflammation in animals (24, 25) and pulmonary epithelial cells in vitro (26). Likewise, heme oxygenase protects against viral infection and replication in cultured human airway epithelial cells (13). Viral infections may induce heme oxygenase in a variety of cell types, including airway epithelial cells and macrophages (27) via the induction of proinflammatory cytokines (7, 8) and NO (11). The increased heme oxygenase activity may then serve to limit the virus infection by inhibiting viral replication (13) and airway inflammation by anti-inflammatory actions (24-26). Because inhaled corticosteroids inhibit the increase in exhaled CO in asthmatic patients (5), corticosteroids may impair host defenses against the viral infection through downregulation of heme oxygenase activity. However, Farr and colleagues (28) reported that the trend toward less increase in nasal obstruction, middle ear pressure, mucus production, and nasal mucus kinin and albumin concentrations during the first 2 d after rhinovirus inoculation was temporally related to the simultaneous administration of oral prednisone and intranasal beclomethasone. Furthermore, dexamethasone inhibits rhinovirus-induced production of cytokines and intercellular adhesion molecule-1 productions and replication of rhinovirus in the cultured human airway epithelial cells in vitro (29). Therefore, corticosteroids may have effects on upper respiratory tract infections through several mechanisms, and a further study is needed to clarify this issue. Furthermore, the administration of NO synthease inhibitors by nebulization to normal subjects and patients with asthma produces a fall in exhaled NO levels (30). Therefore, NO synthase inhibitors may downregulate heme oxygenase activity, thereby decreasing the exhaled CO.
Although we have shown an elevation of exhaled CO in URTIs that decreases after recovery, it is uncertain whether the level of CO in exhaled air is merely an indicator of airway inflammation or a causative link in the biology of airway viral infection. However, the demonstration that URTIs are associated with a high level of exhaled CO suggests that respiratory viral infections may induce the expression of heme oxygenase in the airway. Heme oxygenase therefore may act as a host defense against URTIs.
Because upper respiratory tract infections (URTIs) are reported to induce increases in interleukin (IL)-1β, IL-6, and tumor necrosis factor-α in nasal lavage fluid (14) as well as increases in exhaled NO (15), it is likely that viral respiratory tract infection increases CO production. We have, therefore, studied whether URTIs increase the concentration of CO in the exhaled air of normal persons.
https://www.atsjournals.org/doi/10.1164/ajrccm.158.1.9711066
Received: November 14, 1997
Viral infection may induce the expression of heme oxygenase, resulting in increased carbon monoxide (CO) formation. CO may be produced by various cells of the upper and lower respiratory tract and may be detected in the exhaled air. Therefore, exhaled CO concentrations were measured on a CO monitor by vital capacity maneuver in subjects with upper respiratory tract infections (URTIs) and in nonsmoking and smoking healthy control subjects. At the time of symptoms of URTI, exhaled CO concentrations were 5.6 ± 0.4 ppm and decreased to 1.0 ± 0.1 ppm during recovery. Recovery values of exhaled CO were similar to those in age-matched nonsmoking healthy control subjects (1.2 ± 0.3 ppm). Smoking healthy control subjects had the highest levels of exhaled CO concentration among the groups (18.5 ± 2.5 ppm). These findings suggest that symptomatic URTIs increase the concentration of CO in exhaled air. This may reflect the induction of heme oxygenase that has an antiviral effect in the airways.
Carbon monoxide (CO) has been reported to have several biologic actions (1-4) and may play a role in pathophysiology of airway diseases (5). CO is made by an enzyme called heme oxygenase, and two forms of heme oxygenase have been characterized (6). Constitutive forms of the enzyme are widely distributed throughout the body with high concentrations in the brain (6), but another isoform is induced in several types of cells after exposure to inflammatory cytokines (7, 8), oxidants (9, 10) and NO (11). CO is detectable in the exhaled air of normal persons (5, 12) and exhaled CO is increased in asthma (5), which may reflect an expression of inducible heme oxygenase in airway epithelial cells (13). This is supported by the fact that inhaled corticosteroids inhibit the increase in exhaled CO in asthmatic patients (5).
DISCUSSION
The present study has shown that exhaled CO can be reliably measured in healthy control subjects and subjects during the acute phase of URTIs. The values of exhaled CO in nonsmoking and smoking control subjects were similar to those of previous studies (12, 21, 22). Because the carboxyhemoglobin level in the blood declines exponentially and becomes normal 24 h after the cessation of smoking (23), the present study should underestimate the exhaled CO concentration in smoking control subjects. However, we have demonstrated that URTIs are associated with an increase in exhaled CO in normal persons in the acute phase when symptoms are present, and that there is a reduction in exhaled CO after recovery to values that are similar to those in age-matched normal subjects. This suggests that URTI, presumably caused by influenza viral infection, increases the production of CO in the respiratory tract.
However, several factors might influence the exhaled CO concentration in the present study. First, some variations in the exhaled CO concentration among three sequential maneuvers were observed. Although the reason for observed variations is uncertain, the mean exhaled CO concentrations were similar among three sequential maneuvers in nonsmoking control subjects and the subjects during and after URTIs. Furthermore, the variation between the exhaled CO concentrations on separate days in nonsmoking control subjects was small. Second, the subjects exposed to cigarette smoke as passive smokers might have the same range as the subjects with URTIs. However, it seems unlikely because we selected the subjects with URTIs and none of them were smokers, ex-smokers, or passive smokers. Finally, the subjects might encounter much higher ambient CO levels than those at the place of the CO measurement on their way to the hospital. However, the route and means of transportation to the hospital were the same in the subjects with URTIs between the time of the acute phase and after 3 wk of recovery, and they visited the hospital at a similar time of day. Therefore, the ambient CO levels on the way to the hospital could little influence the difference in the exhaled CO concentration between the time of the acute phase and after 3 wk of recovery in the subjects with URTIs.
The exhaled CO concentration was higher without an expiratory resistance used to close the velum during expiration to exclude nasal CO that may leak throughout expiration in the presence of an open velum (20). Therefore, the increase in CO may be derived from both nasal tissues and the lower respiratory tract. It is of interest that a large proportion of subjects with URTIs in this study complained of lower respiratory tract symptoms, including cough and chest pain accentuated by coughing, indicating that the lower respiratory tract was likely to have been involved.
Heme oxygenase plays an important role in the resolution of inflammation in animals (24, 25) and pulmonary epithelial cells in vitro (26). Likewise, heme oxygenase protects against viral infection and replication in cultured human airway epithelial cells (13). Viral infections may induce heme oxygenase in a variety of cell types, including airway epithelial cells and macrophages (27) via the induction of proinflammatory cytokines (7, 8) and NO (11). The increased heme oxygenase activity may then serve to limit the virus infection by inhibiting viral replication (13) and airway inflammation by anti-inflammatory actions (24-26). Because inhaled corticosteroids inhibit the increase in exhaled CO in asthmatic patients (5), corticosteroids may impair host defenses against the viral infection through downregulation of heme oxygenase activity. However, Farr and colleagues (28) reported that the trend toward less increase in nasal obstruction, middle ear pressure, mucus production, and nasal mucus kinin and albumin concentrations during the first 2 d after rhinovirus inoculation was temporally related to the simultaneous administration of oral prednisone and intranasal beclomethasone. Furthermore, dexamethasone inhibits rhinovirus-induced production of cytokines and intercellular adhesion molecule-1 productions and replication of rhinovirus in the cultured human airway epithelial cells in vitro (29). Therefore, corticosteroids may have effects on upper respiratory tract infections through several mechanisms, and a further study is needed to clarify this issue. Furthermore, the administration of NO synthease inhibitors by nebulization to normal subjects and patients with asthma produces a fall in exhaled NO levels (30). Therefore, NO synthase inhibitors may downregulate heme oxygenase activity, thereby decreasing the exhaled CO.
Although we have shown an elevation of exhaled CO in URTIs that decreases after recovery, it is uncertain whether the level of CO in exhaled air is merely an indicator of airway inflammation or a causative link in the biology of airway viral infection. However, the demonstration that URTIs are associated with a high level of exhaled CO suggests that respiratory viral infections may induce the expression of heme oxygenase in the airway. Heme oxygenase therefore may act as a host defense against URTIs.
Because upper respiratory tract infections (URTIs) are reported to induce increases in interleukin (IL)-1β, IL-6, and tumor necrosis factor-α in nasal lavage fluid (14) as well as increases in exhaled NO (15), it is likely that viral respiratory tract infection increases CO production. We have, therefore, studied whether URTIs increase the concentration of CO in the exhaled air of normal persons.
https://www.atsjournals.org/doi/10.1164/ajrccm.158.1.9711066