Skullcap, Chinese and American, and Thyme.
View: https://twitter.com/DeinertDoc/status/1522135327527292928
View: https://twitter.com/DeinertDoc/status/1522135327527292928
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.

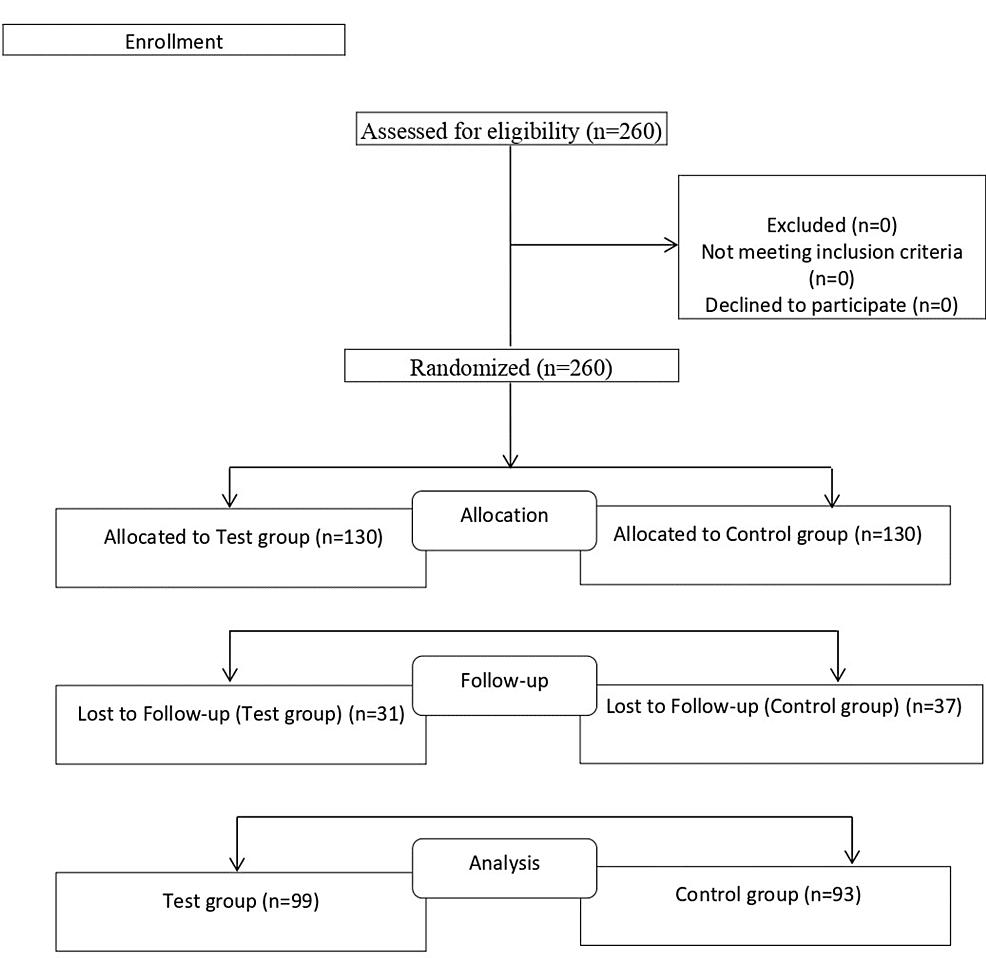
Introduction
In the present study, the combination of two tablets, one with Aspirin and Promethazine and the other with vitamin D3, C, and B3 along with zinc and selenium supplementation was proposed as an intervention (APMV2020). The ingredients in the formulation represent a precise, tailored therapy for the symptoms of COVID-19, combined with natural constituents to help the body itself build immunity to recover from infection. The present study was conducted to clinically validate the safety and efficacy of the APMV2020 tablets.
Trial design
The present trial is a randomized, multicentric, controlled clinical trial involving 260 mild to moderate COVID-19 patients. The treatment duration was of 10 days.
Methodology
The subjects were randomized to receive either the control intervention (clinical management protocol for COVID-19 advocated by the Indian Council of Medical Research (ICMR) or the test intervention (treatment with APMV2020 tablets along with the standard control treatment. The assessment days were baseline, days five and 10.
Results
APMV2020 significantly (<0.05) improved symptoms of COVID-19 like cough, myalgia, headache, and anosmia as compared to the control group. APMV2020 treatment also reduced inflammatory markers like lactate dehydrogenase (LDH), ferritin, and C-reactive protein (CRP).
Conclusion
APMV2020 can prove as a good candidate to be integrated into the COVID-19 management protocol. As it can offer speedy clinical recovery to reduce the burden on healthcare infrastructure, second, the combination shows significant anti-inflammatory potential to improve prognosis, and lastly, the immunomodulatory properties offer long-term protection that can help in combating long COVID symptoms and complications.
Promethazine is great stuff for nausea. Sounds like a Greek hero also.Promethazine

Very interesting ( @Rinse & rePeat is a frequent nettle user)
Carbohydrate-Binding Protein from Stinging Nettle as Fusion Inhibitor for SARS-CoV-2 Variants of Concern
Carbohydrate-Binding Protein from Stinging Nettle as Fusion Inhibitor for SARS-CoV-2 Variants of Concern
Urtica dioica agglutinin (UDA) is a carbohydrate-binding small monomeric protein isolated from stinging nettle rhizomes. It inhibits replication of a broad range of viruses, including coronaviruses, in multiple cell types, with appealing selectivity. In this work, we investigated the potential...www.biorxiv.org
Abstract
Urtica dioica agglutinin (UDA) is a carbohydrate-binding small monomeric protein isolated from stinging nettle rhizomes. It inhibits replication of a broad range of viruses, including coronaviruses, in multiple cell types, with appealing selectivity. In this work, we investigated the potential of UDA as a broad-spectrum antiviral agent against SARS-CoV-2. UDA potently blocks entry of pseudotyped SARS-CoV-2 in A549.ACE2+-TMPRSS2 cells, with IC50 values ranging from 0.32 to 1.22 µM. Furthermore, UDA prevents viral replication of the early Wuhan-Hu-1 strain in Vero E6 cells (IC50 = 225 nM), but also the replication of SARS-CoV-2 variants of concern, including Alpha, Beta and Gamma (IC50 ranging from 115 to 171 nM). In addition, UDA exerts antiviral activity against the latest circulating Delta and Omicron variant in U87.ACE2+ cells (IC50 values are 1.6 and 0.9 µM, respectively). Importantly, when tested in Air-Liquid Interface (ALI) primary lung epithelial cell cultures, UDA preserves antiviral activity against SARS-CoV-2 (20A.EU2 variant) in the nanomolar range. Surface plasmon resonance (SPR) studies demonstrated a concentration-dependent binding of UDA to the viral spike protein of SARS-CoV-2, suggesting interference of UDA with cell attachment or subsequent virus entry. Moreover, in additional mechanistic studies with cell-cell fusion assays, UDA inhibited SARS-CoV-2 spike protein-mediated membrane fusion. Finally, pseudotyped SARS-CoV-2 mutants with N-glycosylation deletions in the S2 subunit of the spike protein remained sensitive to the antiviral activity of UDA. In conclusion, our data establish UDA as a potent and broad-spectrum fusion inhibitor for SARS-CoV-2.
Heliyon
Nutritional and pharmacological importance of stinging nettle (Urtica dioica L.): A review
Stinging nettle (Urtica dioica L.) is a wild herbaceous perennial blooming plant that is commonly known as stinging nettle. It’s a common, multi-purpo…www.sciencedirect.com
Volume 8, Issue 6, June 2022, e09717
Journal home page for Heliyon
Review article
Nutritional and pharmacological importance of stinging nettle (Urtica dioica L.): A review
Abstract
Stinging nettle (Urtica dioica L.) is a wild herbaceous perennial blooming plant that is commonly known as stinging nettle. It’s a common, multi-purpose crop that’s sometimes overlooked. Europe, Asia, North Africa, and North America are all home to stinging nettle. It is a plant that’s edible and has nutritional and medicinal properties. Young leaves can be used to make curries, herb soups, and sour soups. The root of the stinging nettle is used to treat mictional difficulties associated with benign prostatic hyperplasia, while the leaves are used to treat arthritis, rheumatism, and allergic rhinitis. Its leaves are abundant in fiber, minerals, vitamins, and antioxidant compounds like polyphenols and carotenoids, as well as antioxidant compounds like polyphenols and carotenoids. Stinging nettle has antiproliferative, anti-inflammatory, antioxidant, analgesic, anti-infectious, hypotensive, and antiulcer characteristics, as well as the ability to prevent cardiovascular disease, in all parts of the plant (leaves, stems, roots, and seeds). Stinging nettle improves fish reproductive performance, making it a cost-effective aquaculture plant. Fertilizer and insecticides can be made from the plants. This review examines the nutritional and pharmacological aspects of stinging nettle, as well as its possible health advantages. Scientists, farmers, and academicians interested in stinging nettle collecting, cultivation, research, and development would find this review useful.
Virology
Research Article
17 February 2021
Share on
In Vitro Characterization of the Carbohydrate-Binding Agents HHA, GNA, and UDA as Inhibitors of Influenza A and B Virus Replication
ABSTRACT
Here, we report on the anti-influenza virus activity of the mannose-binding agents Hippeastrum hybrid agglutinin (HHA) and Galanthus nivalis agglutinin (GNA) and the (N-acetylglucosamine)n-specific Urtica dioica agglutinin (UDA). These carbohydrate-binding agents (CBA) strongly inhibited various influenza A(H1N1), A(H3N2), and B viruses in vitro, with 50% effective concentration values ranging from 0.016 to 83 nM, generating selectivity indexes up to 125,000. Somewhat less activity was observed against A/Puerto Rico/8/34 and an A(H1N1)pdm09 strain. In time-of-addition experiments, these CBA lost their inhibitory activity when added 30 min postinfection (p.i.). Interference with virus entry processes was also evident from strong inhibition of virus-induced hemolysis at low pH. However, a direct effect on acid-induced refolding of the viral hemagglutinin (HA) was excluded by the tryptic digestion assay. Instead, HHA treatment of HA-expressing cells led to a significant reduction of plasma membrane mobility. Crosslinking of membrane glycoproteins, through interaction with HA, could also explain the inhibitory effect on the release of newly formed virions when HHA was added at 6 h p.i. These CBA presumably interact with one or more N-glycans on the globular head of HA, since their absence led to reduced activity against mutant influenza B viruses and HHA-resistant A(H1N1) viruses. The latter condition emerged only after 33 cell culture passages in the continuous presence of HHA, and the A(H3N2) virus retained full sensitivity even after 50 passages. Thus, these CBA qualify as potent inhibitors of influenza A and B viruses in vitro with a pleiotropic mechanism of action and a high barrier for viral resistance.
FUTURE VIROLOGY VOL. 6, NO. 5 REVIEW
Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy
Dale L Barnard & Yohichi Kumaki
Published Online:24 May 2011 https://doi.org/10.2217/fvl.11.33
Abstract
Severe acute respiratory syndrome coronavirus (SARS-CoV) emerged in early 2003 to cause a very severe acute respiratory syndrome, which eventually resulted in a 10% case-fatality rate. Owing to excellent public health measures that isolated focus cases and their contacts, and the use of supportive therapies, the epidemic was suppressed to the point that further cases have not appeared since 2005. However, despite intensive research since then (over 3500 publications), it remains an untreatable disease. The potential for re-emergence of the SARS-CoV or a similar virus with unknown but potentially serious consequences remains high. This is due in part to the extreme genetic variability of RNA viruses such as the coronaviruses, the many animal reservoirs that seem to be able host the SARS-CoV in which reassortment or recombination events could occur and the ability coronaviruses have to transmit relatively rapidly from species to species in a short period of time. Thus, it seems prudent to continue to explore and develop antiviral chemotherapies to treat SARS-CoV infections. To this end, the various efficacious anti-SARS-CoV therapies recently published from 2007 to 2010 are reviewed in this article. In addition, compounds that have been tested in various animal models and were found to reduce virus lung titers and/or were protective against death in lethal models of disease, or otherwise have been shown to ameliorate the effects of viral infection, are also reported.
....
Inhibiting virus attachment by binding to the virus may be another way of preventing a SARS infection in vivo. Stinging nettle lectin, UDA, is an N-acetyl glucosamine-specific lectin that was reported to be a potent and selective inhibitor of the SARS-CoV strain Frankfurt-1 [84]. Plant lectins like UDA probably target viral attachment and fusion, and exocytosis or egress of the virus from the cell [110]. UDA treatment (mg/kg/day) of BALB/c mice infected with a lethal mouse-adapted strain of Urbani (v2163) resulted in 50% protection from death up to 10 days after infection but no reduction in lung virus titer [85]. In addition, reduction of IL-6 in lungs of mice treated at day 3 post-virus exposure was detected, in contrast with infected mice in which IL-6 levels were extremely high at day 3 post-virus infection [85]. A replicate 21-day study showed that when mice were treated with 15 mg/kg/day, 40% protection from death was achieved with no measurable toxicity.
....
Antiviral Res. 2011 Apr; 90(1): 22–32.
Published online 2011 Feb 19. doi: 10.1016/j.antiviral.2011.02.003
Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin
Abstract
Urtica dioica agglutinin (UDA) is a small plant monomeric lectin, 8.7 kDa in size, with an N-acetylglucosamine specificity that inhibits viruses from Nidovirales in vitro. In the current study, we first examined the efficacy of UDA on the replication of different SARS-CoV strains in Vero 76 cells. UDA inhibited virus replication in a dose-dependent manner and reduced virus yields of the Urbani strain by 90% at 1.1 ± 0.4 μg/ml in Vero 76 cells. Then, UDA was tested for efficacy in a lethal SARS-CoV-infected BALB/c mouse model. BALB/c mice were infected with two LD50 (575 PFU) of virus for 4 h before the mice were treated intraperitoneally with UDA at 20, 10, 5 or 0 mg/kg/day for 4 days. Treatment with UDA at 5 mg/kg significantly protected the mice against a lethal infection with mouse-adapted SARS-CoV (p < 0.001), but did not significantly reduce virus lung titers. All virus-infected mice receiving UDA treatments were also significantly protected against weight loss (p < 0.001). UDA also effectively reduced lung pathology scores. At day 6 after virus exposure, all groups of mice receiving UDA had much lower lung weights than did the placebo-treated mice. Thus, our data suggest that UDA treatment of SARS infection in mice leads to a substantial therapeutic effect that protects mice against death and weight loss. Furthermore, the mode of action of UDA in vitro was further investigated using live SARS-CoV Urbani strain virus and retroviral particles pseudotyped with SARS-CoV spike (S). UDA specifically inhibited the replication of live SARS-CoV or SARS-CoV pseudotyped virus when added just before, but not after, adsorption. These data suggested that UDA likely inhibits SARS-CoV infection by targeting early stages of the replication cycle, namely, adsorption or penetration. In addition, we demonstrated that UDA neutralizes the virus infectivity, presumably by binding to the SARS-CoV spike (S) glycoprotein. Finally, the target molecule for the inhibition of virus replication was partially characterized. When UDA was exposed to N-acetylglucosamine and then UDA was added to cells just prior to adsorption, UDA did not inhibit the virus infection. These data support the conclusion that UDA might bind to N-acetylglucosamine-like residues present on the glycosylated envelope glycoproteins, thereby preventing virus attachment to cells.
Journal of Biological Chemistry
Glycobiology and Extracellular Matrices
Carbohydrate-binding Agents Cause Deletions of Highly Conserved Glycosylation Sites in HIV GP120: A NEW THERAPEUTIC CONCEPT TO HIT THE ACHILLES HEEL OF HIV
Mannose-binding proteins derived from several plants (i.e. Hippeastrum hybrid and Galanthus nivalis agglutinin) or prokaryotes (i.e. cyanovirin-N) inh…www.sciencedirect.com
Carbohydrate-binding Agents Cause Deletions of Highly Conserved Glycosylation Sites in HIV GP120: A NEW THERAPEUTIC CONCEPT TO HIT THE ACHILLES HEEL OF HIV
Mannose-binding proteins derived from several plants (i.e. Hippeastrum hybrid and Galanthus nivalis agglutinin) or prokaryotes (i.e. cyanovirin-N) inhibit human immunodeficiency virus (HIV) replication and select for drug-resistant viruses that show profound deletion of N-glycosylation sites in the GP120 envelope (Balzarini, J., Van Laethem, K., Hatse, S., Vermeire, K., De Clercq, E., Peumans, W., Van Damme, E., Vandamme, A.-M., Bolmstedt, A., and Schols, D. (2004) J. Virol. 78, 10617-10627; Balzarini, J., Van Laethem, K., Hatse, S., Froeyen, M., Van Damme, E., Bolmstedt, A., Peumans, W., De Clercq, E., and Schols, D. (2005) Mol. Pharmacol. 67, 1556-1565). Here we demonstrated that the N-acetylglucosamine-binding protein from Urtica dioica (UDA) prevents HIV entry and eventually selects for viruses in which conserved N-glycosylation sites in GP120 were deleted. In contrast to the mannose-binding proteins, which have a 50-100-fold decreased antiviral activity against the UDA-exposed mutant viruses, UDA has decreased anti-HIV activity to a very limited extent, even against those mutant virus strains that lack at least 9 of 22 (∼40%) glycosylation sites in their GP120 envelope. Therefore, UDA represents the prototype of a new conceptual class of carbohydrate-binding agents with an unusually specific and targeted drug resistance profile. It forces HIV to escape drug pressure by deleting the indispensable glycans on its GP120, thereby obligatorily exposing previously hidden immunogenic epitopes on its envelope.


Yes, I forgot to mention that the tea made from leaves doesn't cut for it, although its anti-histaminic effect might be helpful after infection. I tried green tea for its high quercetin content, but the tea is probably too immunostimulant and made symptoms worse.Thanks for the study. It is the root (rhizome) of the nettle plant and not the leaves. Health shops sell both separately.

Urtica dioica agglutinin. A superantigenic lectin from stinging nettle rhizome - PubMed
Urtica dioica agglutinin (UDA) is an unusual plant lectin that differs from all other known plant lectins with respect to its molecular structure and its extremely low specific agglutination activity. We recently reported that this small lectin (8.5 kDa) is a T cell mitogen distinguishable from...pubmed.ncbi.nlm.nih.gov
The root has high contents of glycine, cysteine and tryptophan.

An unusual lectin from stinging nettle (Urtica dioica) rhizomes
An unusual lectin has been isolated from stinging nettle (Urtica dioica L.) rhizomes. It is a small (8.5 kDa) monomeric protein with high contents of …www.sciencedirect.com

 www.ukcolumn.org
www.ukcolumn.org


Nice @Zsazsa. Thank you.
Acetylsalicylic acid and Salicylic acid inhibit SARS-CoV-2 replication in precision-cut lung slices
Aspirin, with its active compound acetylsalicylic acid (ASA), shows antiviral activity against rhino- and influenza viruses and at high concentrations. We sought to investigate whether ASA and its metabolite salicylic acid (SA) inhibit SARS-CoV-2 since it might use similar pathways to influenza...www.biorxiv.org

Vitamin B12 attenuates leukocyte inflammatory signature in COVID-19 via methyl-dependent changes in epigenetic marks
COVID-19 induces chromatin remodeling in host immune cells, and it had previously been shown that vitamin B12 downregulates some inflammatory genes via methyl-dependent epigenetic mechanisms. In this work, whole blood cultures from moderate or severe COVID-19 patients were used to assess the...www.biorxiv.org