SB4
Member
- Joined
- Sep 25, 2016
- Messages
- 288
It's based on the studies done on the cigniture health website (this is where I got saliva test, still waiting on results). 1/3 25ppm + 2/3 155ppm = 108ppm so close to what they use on some studies. Perhaps it would, I have seen an interview where one doctor says he uses it to offset his crap eating.Interesting. How did you come up with that ratio?
Is it only UV light that removes deuterium? Or could red light help?
So if one is doing the peaty thang and drinking 2 quarts each of (sea level) milk and oj, plus lots of deut-heavy carbs, would 1/2 liter of light water make a difference?
Where can one buy a salivary deuterium test?
O chemists, what do you think of this simple diy method for light water?
On another forum they determined that it would only remove a very small amount of D and considering that all the labs use very expensive ways to make DDW, I think this is a safe bet.
I have no idea how it can choose to put D in some places and not others but its fascinating. Perhaps it can somehow use enzymes to test if the H is D or not by how quick it reacts? Perhaps this is where UV comes in, if one consumes a lot of D in the Carbon 2 position perhaps UV can kick it off somehow, making carbs + sun = D depleted TCA?That's a neat article, and it's about ²H only. Carbon‐13 is much less abundant but has less kinetic isotope effects because it only weighs ~8% more than ¹²carbon (¹³⁄₁₂ = 8.333%), while deuterium weighs 100% more than hydrogen. The kinetic isotope effects of carbon‐13 can be measured but they are negligible relative to hydrogen's.
The most interesting thing about the article, in my opinion, is this passage:
'More specifically, natural glucose source isolated from leaf starch of common bean (Phaseolus vulgaris) or spinach (Spinacia oleracea) is depleted in deuterium in the C(2) position. Carbon specific deuterium depletion in fatty acids from plants [42] and other sources [43,44] is also evident, which generate deuterium depleted matrix water in mitochondria during complete oxidation in complex-IV.' ―László G Boros
You would think that ²H and ¹H would exist throughout the entire glucose molecule at random: ~155‧ppm everywhere, with the odds of any one hydrogen having a neutron being .0155%—completely without carbon‐to‐carbon variation. But the carbon #2 of the bean‐derived glucose is apparently deuterium‐depleted on just that one carbon [?], which is puzzling. Perhaps gravity is working opposite capillary action in the bean stalk? separating the lighter ¹H₂O from the deuterated water as its pulled down by gravity, producing a distribution in which ²H₂O is tending more towards the lower half of the stem?
And it looks as though the NADH ⟶ NAD⁺ kinetic isotope effects are considerable; this is the main cofactor for these hydride [:H] transfer reactions. At body temperature (94.73°F; 308‧K), the hydride transfer reaction of an NADH analogue was measured as having kinetic isotope effect of 14:
View attachment 8163
The cofactor NADH has two hydrogen atoms on its catalytic carbon—despite only one being written there—so it could potentially exist as a mixed species having one ²H and one ¹H. In this case, a person could be interested in knowing if it would just selectively transfer the lighter ¹H at the very same rate as an NADH molecule would having two light hydrogens. This would be a fair question, and I think the answer would be 'no.'
View attachment 8164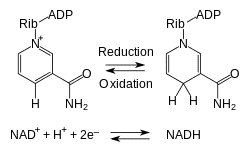
Since the catalytic carbon of NADH is sp³‐hybridized, the hydrogens stay to one side of the molecule—they do not interconvert. The hydrogen on one face of the molecule must remain in that position until either itself or the other hydrogen is removed—after which that carbon becomes sp²‐hybridized, non‐optically‐active, symmetrical, and planar. And since NADH exists in enzymatic binding sites in only one orientation, you would expect only one hydrogen on that carbon to be 'active'—or transferable—since only one hydrogen would be facing the substrate. So an NADH molecule with only one deuterium, as long as its on the correct side of the ring, would be expected to transfer at a slower rate (~14×); there is no need to assume that NADH needs both hydrogens to be deuterium—a statistically improbably event. At normal enrichment, the chance of both NADH hydrogens being deuterium is only .0000024%.
So the kinetic isotope effect of about 14 seems to be a fair number for NADH hydride transfers; but considering the fact that NADH is often stabilized inside of an enzyme could change this number. Perhaps we need to look for at a kinetic isotope study on NADH metabolic enzymes . . . if there is one?
Lu, Yun. "Hydride-exchange reactions between NADH and NAD⁺ model compounds under non-steady-state conditions. Apparent and real kinetic isotope effects." Organic & biomolecular chemistry (2003)
For the spinach it is assumed that as D is pro growth, the fruits and seeds of the plant have high D whilst the leafy green low D. This kills 2 birds with one stone as baby plant has enough D to grow whilst adult plant has low D so mito can run faster.
Not all of the hydrogens exit as acidic protons (H⁺), or as proton–neutrons in the case of ionized deuterium (D⁺). Some of these end‐up as H₂O, being transferred to O₂ by heme‐catalyzed enzymes at the very end of the 'electron transport chain.' The electrons flow through microtubules to distant places where they eventually should flow through a heme complex and then discharged; they flow to the center of a porphyrin ring, convert Fe³⁺ to Fe²⁺, and this heme‐ligated iron(II) atom then adsorbs an O₂ molecule:
[1] Fe²⁺–Ö–Ö:⁻
This then attracts a proton (H⁺) from solution—likely from hydronium (H₃O⁺):
[2] Fe²⁺–Ö–Ö:H
And more electrons are then collected by the heme complex and funneled to iron, electrons (e⁻) which are then donated to the oxygen species adsorbed onto iron. This sequential reduction eventually ends with two H₂O molecules for every one O₂:
[3] Fe³⁺–Ö:⁻ + ⁻Ö:H
[4] Fe²⁺–Ö:H + H:Ö:H
[5] Fe³⁺ + ⁻Ö:H + H₂O
[6] Fe³⁺ + H:Ö:H + H₂O
[7] Fe³⁺ + H₂O + H₂O
[8] Fe²⁺ + 2‧H₂O
Very interesting, thanks, this has cleared up some things for me.
