Mito
Member
- Joined
- Dec 10, 2016
- Messages
- 2,554
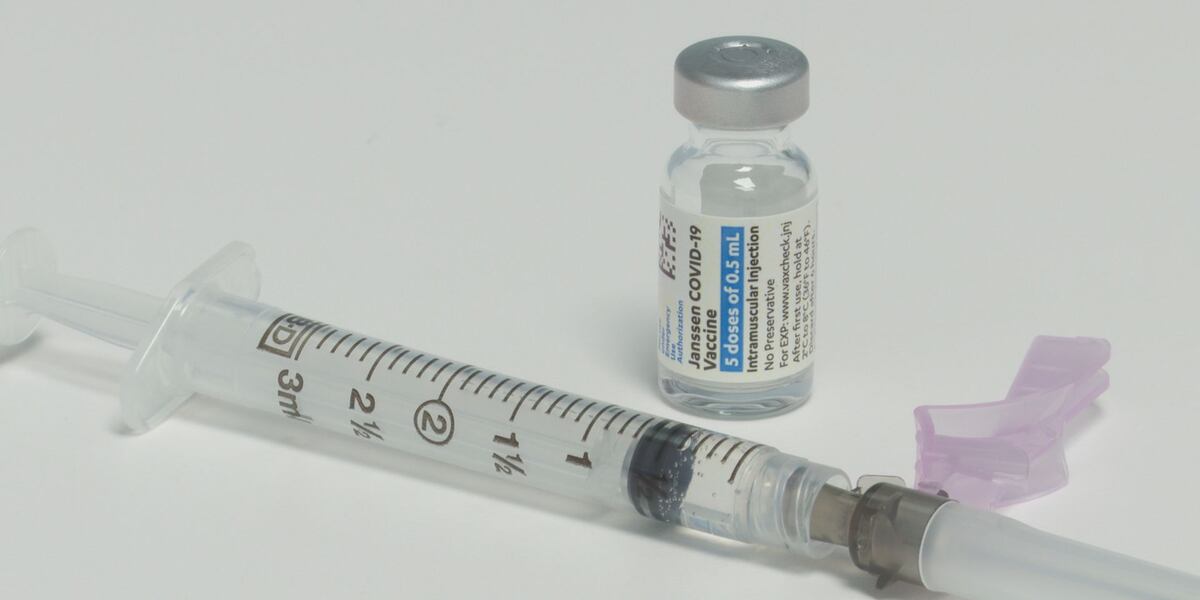
“The U.S. Centers for Disease Control & Prevention is looking into a Virginia woman’s death as part of its investigation into possible adverse side effects from the Johnson & Johnson COVID-19 vaccine. According to the state’s vaccine coordinator, Dr. Danny Avula, the woman’s death happened in March and was reported to the CDC’s Vaccine Adverse Event Reporting System (VAERS). On Tuesday, the CDC and FDA called for a pause in the use of the Johnson & Johnson vaccine after six women developed a rare condition involving blood clots after receiving the vaccine. “This pause was recommended out of an abundance of caution, as these adverse events appear to be extremely rare. To date, more than 6.8 million people in the United States have received Johnson & Johnson vaccines and six recipients are known to have developed a type of blood clot called cerebral venous sinus thrombosis (CVST) in combination with low levels of blood platelets (thrombocytopenia),” Avula said in a release. All six cases were among women ages 18 to 48, and symptoms started six to 13 days after vaccination. “CDC will convene a meeting of the Advisory Committee on Immunization Practices (ACIP) on Wednesday, April 14, to further review these cases and assess their potential significance. FDA will review that analysis as it also investigates these cases,” a release said.
Virginia has stopped using the Johnson & Johnson vaccine until the investigation is complete. The state’s vaccine rollout will continue with Pfizer and Moderna.
 www.nbc12.com
www.nbc12.com
Virginia has stopped using the Johnson & Johnson vaccine until the investigation is complete. The state’s vaccine rollout will continue with Pfizer and Moderna.

CDC investigating Virginia woman’s death in connection to possible side-effects from Johnson & Johnson vaccine
The U.S. Centers for Disease Control & Prevention is looking into a Virginia woman’s death as part of its investigation into possible adverse side effects from the Johnson & Johnson COVID-19 vaccine.