Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
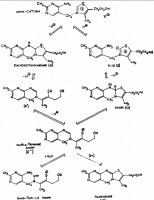
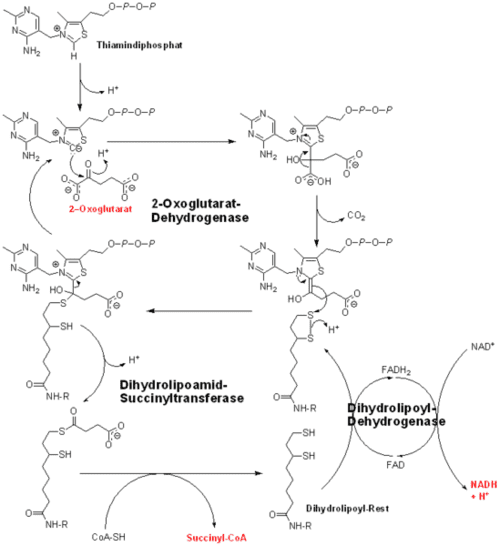
The orthodox reaction model (Brezlow) is shown below from Wikipedia. Coenzyme A can be seen taking the acetyl group from the thiamine complex. Notable is that there is no opening of the thiazole ring in the Breslow mechanism.
However, A.J. Knell has some things to say about this mechanism:
6. Breslow's theory is unsatisfactory for the following reasons.
(a) Intermolecular reaction at the 2'-position is stercially hindered, and it is difficult or impossible to build space-filling models of the proposed intermediates.
(b) It accounts for only the first of the structural features essential for activity
(c) It does not easily explain the oxidative functions of thiamine.
(d) 2'-(1"-Hydroxyethyl)thlamine pyrophosphate Is not a cofactor for apo-pyruvate decarboxylase (table 1), and the 1"-proton exchanges slowly. 2'-Acetyl thiazollum salts are notacetylating agents in water.
(e) It is difficult to show that thiamine acts catalytically in the model reaction, and the results obtained using thiamine analogues differ qualitatively and quantitatively from the results of enzyme studies
He goes to show his proposed mechanism of thiamine decarboxylation. This is radically different and interesting:



He justifies it with evidence and logic:
11. The advantages of this theory are as follows:
(a) There are no steric objections.
(b) It accounts for most of the observations relating to structure and function.
(c) The oxidative function of thiamine is explained (figure 8). The intermediate (15) should be easily oxidised, by analogy with the oxidation of xantho-thiamine and dihydrothiochrome to thiochrome. The product is an S-acyl xantho-thiamine (16). Thiol esters are usually reactive, and S-acyl leuco-thiamine (17) will transfer the acyl group to the S'-(2''-hydroxy)ethyl group or to solvent.
(d) It is consistent with the results of studies of the active sites.
This is a neat 144-page thesis on thiamine with a wealth of information. The author is racy and is not afraid to b****-slap other people's conclusions.
I looked-up the author and he (ostensibly) got his PhD for writing this. He later did studies on billirubin and disappeared from the public record.
Nonetheless, this is a cool thesis with 191 references. Besides chemical and structural data, there are sections on enzymes.
THIAMINE: A Study of its Chemistry, Biochemistry and Mechanism of Action

However, A.J. Knell has some things to say about this mechanism:
6. Breslow's theory is unsatisfactory for the following reasons.
(a) Intermolecular reaction at the 2'-position is stercially hindered, and it is difficult or impossible to build space-filling models of the proposed intermediates.
(b) It accounts for only the first of the structural features essential for activity
(c) It does not easily explain the oxidative functions of thiamine.
(d) 2'-(1"-Hydroxyethyl)thlamine pyrophosphate Is not a cofactor for apo-pyruvate decarboxylase (table 1), and the 1"-proton exchanges slowly. 2'-Acetyl thiazollum salts are notacetylating agents in water.
(e) It is difficult to show that thiamine acts catalytically in the model reaction, and the results obtained using thiamine analogues differ qualitatively and quantitatively from the results of enzyme studies
He goes to show his proposed mechanism of thiamine decarboxylation. This is radically different and interesting:
He justifies it with evidence and logic:
11. The advantages of this theory are as follows:
(a) There are no steric objections.
(b) It accounts for most of the observations relating to structure and function.
(c) The oxidative function of thiamine is explained (figure 8). The intermediate (15) should be easily oxidised, by analogy with the oxidation of xantho-thiamine and dihydrothiochrome to thiochrome. The product is an S-acyl xantho-thiamine (16). Thiol esters are usually reactive, and S-acyl leuco-thiamine (17) will transfer the acyl group to the S'-(2''-hydroxy)ethyl group or to solvent.
(d) It is consistent with the results of studies of the active sites.
This is a neat 144-page thesis on thiamine with a wealth of information. The author is racy and is not afraid to b****-slap other people's conclusions.
I looked-up the author and he (ostensibly) got his PhD for writing this. He later did studies on billirubin and disappeared from the public record.
Nonetheless, this is a cool thesis with 191 references. Besides chemical and structural data, there are sections on enzymes.
THIAMINE: A Study of its Chemistry, Biochemistry and Mechanism of Action
Last edited: