Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
-
By using this site you agree to the terms, rules, and privacy policy.
-
Charlie's Restoration Giveaway #2 (Entire Home EMF Mitigation & Protection Along With Personal Protection) - Click Here To Enter
-
Dear Carnivore Dieters, A Muscle Meat Only Diet is Extremely Healing Because it is a Low "vitamin A" Diet. This is Why it Works so Well...
Rest the rest of this post by clicking here
-
The Forum is transitioning to a subscription-based membership model - Click Here To Read
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Amino Acid Supplementation For People With Poor Digestion
- Thread starter haidut
- Start date
Hgreen56
Member
- Joined
- Apr 8, 2020
- Messages
- 723
I was thinking this to.So it means that dairy is a bad source of protein because there is a lot of nitrogen metabolites from it, hence some people can't tolerate it?
Can someone confirm? @haidut @Hans @Amazoniac
- Joined
- Aug 24, 2017
- Messages
- 5,856
This is most likely not the case. If someone was sensitive to the nitrogen metabolites, then they would be sensitive to most protein sources, not just from dairy. Most people struggle with A1 casein, but a minority also can't tolerance whey.
Hgreen56
Member
- Joined
- Apr 8, 2020
- Messages
- 723
thanksThis is most likely not the case. If someone was sensitive to the nitrogen metabolites, then they would be sensitive to most protein sources, not just from dairy. Most people struggle with A1 casein, but a minority also can't tolerance whey.
Amazoniac
Member
"The mean protein concentration of animal tissues (16.5%) is nearly twice as high as that of plants and contains significantly larger proportions of the two main S-containing amino acids (SAAs) (4.48% per gram of protein), with Met (3.17%) prevailing over Cys (1.31%), which accounts for the higher ratios of Met:Cys in animal protein products than in plant protein products.[8; last here] Although the tissue distributions of S and N compounds are not strictly aligned and minor fractions of TBS participate in the structure of lipid and carbohydrate molecules, mammalian tissues (rat, dog, cattle, human) nevertheless manifest large homogeneity in terms of protein and SAA content, with S:N molar ratios usually approximating 1:14.5.[8] Egg white is a notable exception, constituting one of the richest sources of S (S:N ratio, 1:10). The data provided by N and S balance studies indicate that the fate of dietary S is not distinguishable from that of N,[32] implying that the body's capacity to enter into anabolic drive and to generate net tissue protein gain is the requisite determinant of S accretion rate.[33]"
"Under physiological circumstances, kinetic studies using dual-labeled tracer material in healthy adults consuming well-balanced diets with appropriate Met intake levels indicate that both RM [remethylation] and TS [transulfuration] pathways split into nearly equivalent fractions,[96] confirming previous human investigations using total body CH3 balance approaches.[97] In the case of subnormal Met dietary intake, the respective proportion of Hcy fluxes driven toward RM processes increases at the expense of TM [transmethylation] and TS pathways so as to maintain intra- and extracellular Met homeostasis.[77,96,97] The partitioning of Met flow between two converting pathways occurs mainly in the liver and is coordinated by the intracellular concentration of SAM, which works as an inhibitor of both MTHFR and BHMT and as an allosteric activator of CBS activities.[87,90,98] It is worth mentioning that elderly persons may adjust less well to a reduction in the dietary intake of SAAs than do young adults.[99] During the first intestinal pass and before reaching the portal blood flow, dietary SAAs may produce distinct Met and Cys plasma levels, depending on whether administration is oral or parenteral.[100] Animal experiments indicate that gastrointestinal tissues possess all enzymes necessary to achieve TM, RM, and TS activities.[48,92,101] Studies conducted in healthy volunteers indicate that, for an adequate SAA intake, the first pass through splanchnic tissues metabolizes about 23% of dietary Met,[49] part of which is utilized for the synthesis of intraluminal Cys-rich mucins that safeguard the intestinal epithelia[102] and the production of antioxidative compounds.[103] As a result, supranormal Met intake by humans increases TM and TS rates and decreases RM rates in splanchnic tissues,[48] thereby promoting the leakage of Met molecules ingested in excess. The opposite metabolic pattern was observed in pigs submitted to Met-restricted conditions, who showed impairment of SAA degradative pathways contrasting with upregulated RM processes.[104] Taken together, the data indicate that splanchnic organs contribute to the maintenance of adaptive Met homeostasis under conditions of inadequate protein consumption. These Met-salvaging properties are likely compromised in overt protein-depleted states because the intestinal mucosa, characterized by intense turnover rate, undergoes an involutive flattening process proportionate to the degree of protein malnutrition both in piglet models[104] and in humans.[105]"
"N and S maintain tightly correlated ratios in tissues of both healthy subjects[8] and diseased patients.[167] Acute stressful conditions of any cause unleash a shower of many cytokines that fulfill a myriad of autocrine, paracrine, and endocrine functions.[168] As a consequence, enhanced tissue proteolysis throughout the body ensues, allowing the redirection of AA residues toward the preferential overproduction of acute-phase reactants and repair proteins by the liver and at the site of injury.[169] The rate of protein degradation usually exceeds that of protein undergoing neosynthesis,[170,171] leading to a negative N balance with subsequent depletion of TBN reserves. The increased urinary excretion of N catabolites (mainly urea, but also creatinine, NH4+, 3-CH3-histidine, and other minor compounds) demonstrates that both metabolic and structural tissues participate in the adaptive responses to injury in proportion to the magnitude of initial impact.[31,170,171] In very aggressive conditions (burns) affecting adult men, urinary output of N may be as high as 250 g of N per week, which corresponds to a loss of 6–7 kg of LBM[31] or 12–14% of metabolically active tissues.[30] Major stressful disorders are associated with massive urinary excretion of S,[167,172] which depletes endogenous pools of TBS. In stressors of medium severity (bone fracture), S spillover has been estimated to be 17 g of S per week, or more than 10% of TBS body stores. Interestingly enough, measurement of S and N urinary losses yields values very close to the 1:14 ratio that characterizes mammalian tissues,[8,167] indicating that TBN and TBS pools exhibit concomitant degradation patterns throughout the course of injury."
"TTR [Transthyretin/prealbumid] is an exquisitely sensitive biomarker of declining nutritional status[148,149] that accurately reflects LBM fluctuations in health and disease.[30]"
"The initiation of acute injury releases N and S urinary catabolites,[167] whose peak values coincide with the nadir recorded for TTR decline[31] and culminate with Hcy elevation, as shown in coronary heart infarct,[178] acute pancreatitis,[179] and critically ill patients.[180] When the stressful condition subsides and, provided that appropriate nutritional support is offered, N balance and TTR values gradually normalize, allowing Hcy to return to baseline levels within a couple of days.[30,31] Inadequate dietary management and persistence of metabolic or septic complications result in continued N and S urinary losses, identified by subnormal TTR and supranormal Hcy plasma concentrations that maintain distortion patterns diverging from normal concentrations, hence revealing a mirror image of each other.[30,31]"
"Chronic stressful conditions are also characterized by cytokine overproduction of lesser intensity but nevertheless causing comparable adverse effects on LBM integrity. Most low-grade inflammatory conditions are usually characterized by overproduction of C-reactive protein and other acute-phase reactants that act in association with insulin resistance[181] and HHcy,[182] leading to increased risk of CVD. Evidence of LBM downsizing is well documented in kidney patients and diabetics,[183] who excrete around 4 g of albumin per day and who are expected to lose 3.3 kg of LBM (7% of metabolically active tissues,[30]) on a yearly basis. In agreement with the concept of LBM depletion, the occurrence of HHcy appears to be the expected correlate of (micro)albuminuria[184,185] and insulin refractoriness,[186,187] both of which occur during the course of most protracted disorders. Proteinuria reflects the slope of the LBM depletion rate and constitutes an important marker of disease activity and mortality risk,[184,185] as reported in kidney failure,[135] lipoid nephrosis,[188] and eclampsia.[189] Patients suffering from homosexuality,[190] cervical cancer, and breast cancer[191,192] develop an HHcy status of a magnitude that seems proportionate to the rate of cell proliferation and metastatic invasion,[191] thereafter subsiding during successful therapy.[193] Subacute disorders characterized by cyclic bouts with spontaneous relapses or drug-induced remissions usually exhibit up and down alterations of Hcy values in relation to disease activity. Such erratic fluctuations in Hcy values have been documented in autoimmune processes,[194] drepanocytosis,[195] and parasitic infestations.[196]"
"Under physiological circumstances, kinetic studies using dual-labeled tracer material in healthy adults consuming well-balanced diets with appropriate Met intake levels indicate that both RM [remethylation] and TS [transulfuration] pathways split into nearly equivalent fractions,[96] confirming previous human investigations using total body CH3 balance approaches.[97] In the case of subnormal Met dietary intake, the respective proportion of Hcy fluxes driven toward RM processes increases at the expense of TM [transmethylation] and TS pathways so as to maintain intra- and extracellular Met homeostasis.[77,96,97] The partitioning of Met flow between two converting pathways occurs mainly in the liver and is coordinated by the intracellular concentration of SAM, which works as an inhibitor of both MTHFR and BHMT and as an allosteric activator of CBS activities.[87,90,98] It is worth mentioning that elderly persons may adjust less well to a reduction in the dietary intake of SAAs than do young adults.[99] During the first intestinal pass and before reaching the portal blood flow, dietary SAAs may produce distinct Met and Cys plasma levels, depending on whether administration is oral or parenteral.[100] Animal experiments indicate that gastrointestinal tissues possess all enzymes necessary to achieve TM, RM, and TS activities.[48,92,101] Studies conducted in healthy volunteers indicate that, for an adequate SAA intake, the first pass through splanchnic tissues metabolizes about 23% of dietary Met,[49] part of which is utilized for the synthesis of intraluminal Cys-rich mucins that safeguard the intestinal epithelia[102] and the production of antioxidative compounds.[103] As a result, supranormal Met intake by humans increases TM and TS rates and decreases RM rates in splanchnic tissues,[48] thereby promoting the leakage of Met molecules ingested in excess. The opposite metabolic pattern was observed in pigs submitted to Met-restricted conditions, who showed impairment of SAA degradative pathways contrasting with upregulated RM processes.[104] Taken together, the data indicate that splanchnic organs contribute to the maintenance of adaptive Met homeostasis under conditions of inadequate protein consumption. These Met-salvaging properties are likely compromised in overt protein-depleted states because the intestinal mucosa, characterized by intense turnover rate, undergoes an involutive flattening process proportionate to the degree of protein malnutrition both in piglet models[104] and in humans.[105]"
"N and S maintain tightly correlated ratios in tissues of both healthy subjects[8] and diseased patients.[167] Acute stressful conditions of any cause unleash a shower of many cytokines that fulfill a myriad of autocrine, paracrine, and endocrine functions.[168] As a consequence, enhanced tissue proteolysis throughout the body ensues, allowing the redirection of AA residues toward the preferential overproduction of acute-phase reactants and repair proteins by the liver and at the site of injury.[169] The rate of protein degradation usually exceeds that of protein undergoing neosynthesis,[170,171] leading to a negative N balance with subsequent depletion of TBN reserves. The increased urinary excretion of N catabolites (mainly urea, but also creatinine, NH4+, 3-CH3-histidine, and other minor compounds) demonstrates that both metabolic and structural tissues participate in the adaptive responses to injury in proportion to the magnitude of initial impact.[31,170,171] In very aggressive conditions (burns) affecting adult men, urinary output of N may be as high as 250 g of N per week, which corresponds to a loss of 6–7 kg of LBM[31] or 12–14% of metabolically active tissues.[30] Major stressful disorders are associated with massive urinary excretion of S,[167,172] which depletes endogenous pools of TBS. In stressors of medium severity (bone fracture), S spillover has been estimated to be 17 g of S per week, or more than 10% of TBS body stores. Interestingly enough, measurement of S and N urinary losses yields values very close to the 1:14 ratio that characterizes mammalian tissues,[8,167] indicating that TBN and TBS pools exhibit concomitant degradation patterns throughout the course of injury."
"TTR [Transthyretin/prealbumid] is an exquisitely sensitive biomarker of declining nutritional status[148,149] that accurately reflects LBM fluctuations in health and disease.[30]"
"The initiation of acute injury releases N and S urinary catabolites,[167] whose peak values coincide with the nadir recorded for TTR decline[31] and culminate with Hcy elevation, as shown in coronary heart infarct,[178] acute pancreatitis,[179] and critically ill patients.[180] When the stressful condition subsides and, provided that appropriate nutritional support is offered, N balance and TTR values gradually normalize, allowing Hcy to return to baseline levels within a couple of days.[30,31] Inadequate dietary management and persistence of metabolic or septic complications result in continued N and S urinary losses, identified by subnormal TTR and supranormal Hcy plasma concentrations that maintain distortion patterns diverging from normal concentrations, hence revealing a mirror image of each other.[30,31]"
"Chronic stressful conditions are also characterized by cytokine overproduction of lesser intensity but nevertheless causing comparable adverse effects on LBM integrity. Most low-grade inflammatory conditions are usually characterized by overproduction of C-reactive protein and other acute-phase reactants that act in association with insulin resistance[181] and HHcy,[182] leading to increased risk of CVD. Evidence of LBM downsizing is well documented in kidney patients and diabetics,[183] who excrete around 4 g of albumin per day and who are expected to lose 3.3 kg of LBM (7% of metabolically active tissues,[30]) on a yearly basis. In agreement with the concept of LBM depletion, the occurrence of HHcy appears to be the expected correlate of (micro)albuminuria[184,185] and insulin refractoriness,[186,187] both of which occur during the course of most protracted disorders. Proteinuria reflects the slope of the LBM depletion rate and constitutes an important marker of disease activity and mortality risk,[184,185] as reported in kidney failure,[135] lipoid nephrosis,[188] and eclampsia.[189] Patients suffering from homosexuality,[190] cervical cancer, and breast cancer[191,192] develop an HHcy status of a magnitude that seems proportionate to the rate of cell proliferation and metastatic invasion,[191] thereafter subsiding during successful therapy.[193] Subacute disorders characterized by cyclic bouts with spontaneous relapses or drug-induced remissions usually exhibit up and down alterations of Hcy values in relation to disease activity. Such erratic fluctuations in Hcy values have been documented in autoimmune processes,[194] drepanocytosis,[195] and parasitic infestations.[196]"
- Nitrogen and protein content measurement and nitrogen to protein conversion factors for dairy and soy protein-based foods: a systematic review and modelling analysis
"Nitrogen to protein conversion factors (NPCFs) allow for the estimation of protein content in food samples from the amount of nitrogen in the sample, based on two assumptions: that most of the nitrogen is associated with amino acids and that most of the amino acids in foods are associated with protein. The accuracy of the estimation depends on the value of the conversion factor. A value of 6.25 is applied for measuring protein content in most foods and food ingredients, again based on two assumptions: that proteins contain about 16% nitrogen by weight (i.e. of the total mass of a protein, 16% is nitrogen) [6.25 × 16% = 100%], and that all nitrogen in food is derived from protein. However, using the same conversion factor for all protein sources can introduce errors that lead to significant overestimation or underestimation of the actual protein content of most foods. Hence, a default value of 6.25 may not be an appropriate conversion factor for all protein sources; instead, specific values should be considered for different foods and food ingredients."
"The nitrogen content of individual amino acids varies according to the molecular weight of the amino acid and the number of nitrogen atoms it contains (from one to four, depending on the amino acid in question) (Sosulski & Imafidon, 1990). Thus, there are important differences in the nitrogen content of individual amino acids, distributed according to the amount of nitrogen in their residues:
– nitrogen-poor residues contain from 8.58% to 10.85% nitrogen: tyrosine, phenylalanine, methionine and glutamic acid;
– nitrogen-rich residues contain from 21.86% to 35.87% nitrogen: lysine, glutamine, asparagine, glycine, histidine and arginine; and
– the remaining 10 residues have a nitrogen content that ranges from 12.17% (aspartic acid) to 19.7% (alanine)."
"The variations in individual amino acid composition between proteins and in the nitrogen content of individual amino acids mean that the “generic” nitrogen content of protein is not 16%, but in reality can vary from about 13% to 19%. The fact that proteins from diverse sources vary in their nitrogen content owing to differences in their amino acid composition was first demonstrated through protein purification and nitrogen content analysis of extracted purified proteins (Jones, 1931)."
"For many years, the protein content of foods has been determined from total nitrogen content. The protein content in a food is traditionally estimated by multiplying the total nitrogen content by a NPCF, based on the assumptions that dietary carbohydrates and fats do not contain nitrogen, and that most of the nitrogen in the diet is present as amino acids in proteins. However, protein and nitrogen content are defined differently (Jones, 1931; Tkachuk, 1969; Sosulski & Imafidon, 1990; Mossé, 1990; Maubois & Lorient, 2016; Krul, 2019) (Table 1)."
"The conversion factor was historically set at 6.25 by assuming that most, if not all, nitrogen in food was derived from protein, and that the nitrogen content of proteins was about 16%. This approach is still an accepted method for calculating the crude protein content of foods and food ingredients; however, it has been known for decades that using total nitrogen content with a conversion factor of 6.25 to quantify total protein is imperfect and can lead to a 15–20% error in the actual protein content."
"The errors for deriving the crude protein content from nitrogen content have three main origins, as outlined below:
– Total nitrogen content varies between the different amino acids; thus, because amino acid composition varies from one protein source to another, the protein nitrogen content also varies between different protein sources.
– In addition to amino acids, some proteins contain prosthetic groups, some of which are non-nitrogenous and therefore increase the molecular weight of the protein without significantly affecting the nitrogen content. In turn, this results in an increase in the conversion factor, because both amino acids and amino acids plus prosthetic groups are being considered on a weight basis.
– The fraction of non-protein nitrogen varies in different food protein sources; this can result in a lower value of the conversion factor when the total nitrogen content of foods is being considered."
"Newer approaches use values for protein weight and protein nitrogen content of different proteins based on knowledge of the amino acid composition as obtained via amino acid analysis or amino acid sequencing (Heathcote, 1950; Tkachuk, 1969; Holt & Sosulski, 1979; Morr, 1981; Mossé, 1990)." "Calculation of the weight of the protein can include either the sum of the amino acid residues only, or the sum of the amino acid residues plus the weight of the associated prosthetic groups when these are analysed by specific methods or obtained from amino acid sequencing."
"An important feature of approaches based on amino acid composition is the use of the anhydrous weight of amino acids to determine protein weight. Using the free amino acid weight for a protein would grossly overestimate the weight for longer polypeptide chains. Because each amino acid residue in a polypeptide chain loses one water molecule (weighing 18 Da) during polymerization, except for the residue at the carboxy terminus; thus, the weight of the protein must be assessed as the sum of the weight of the anhydrous amino acid residues rather than the weight of the free amino acids (each of which weighs 18 Da more than its anhydrous counterpart)."
"Molecular weights of free and anhydrous forms of all 20 amino acids, and the corresponding percentage of nitrogen, are shown in Table 2."

"Another important consideration when calculating NPCFs relates to the differential contribution to nitrogen content of the amide forms of the amino acids, glutamine and asparagine, and their respective acid forms, glutamic acid and aspartic acid.
With standard amino acid analysis of a protein sample, glutamine and asparagine are converted to glutamic acid and aspartic acid during acid hydrolysis (by substitution of a carboxyl group for the amide group); the total of glutamic acid plus glutamine and aspartic acid plus asparagine is then determined. This conversion does not significantly affect the calculation of the total anhydrous amino acid weight, because there is little difference between the molecular weights of glutamic acid and glutamine (147.13 Da and 146.15 Da, respectively) and aspartic acid and asparagine (133.11 Da and 132.12 Da, respectively). However, failing to consider the ratio between the amide and acid forms induces a major error in the calculation of total nitrogen content of the amino acid residues."
"The nitrogen content of individual amino acids varies according to the molecular weight of the amino acid and the number of nitrogen atoms it contains (from one to four, depending on the amino acid in question) (Sosulski & Imafidon, 1990). Thus, there are important differences in the nitrogen content of individual amino acids, distributed according to the amount of nitrogen in their residues:
– nitrogen-poor residues contain from 8.58% to 10.85% nitrogen: tyrosine, phenylalanine, methionine and glutamic acid;
– nitrogen-rich residues contain from 21.86% to 35.87% nitrogen: lysine, glutamine, asparagine, glycine, histidine and arginine; and
– the remaining 10 residues have a nitrogen content that ranges from 12.17% (aspartic acid) to 19.7% (alanine)."
"The variations in individual amino acid composition between proteins and in the nitrogen content of individual amino acids mean that the “generic” nitrogen content of protein is not 16%, but in reality can vary from about 13% to 19%. The fact that proteins from diverse sources vary in their nitrogen content owing to differences in their amino acid composition was first demonstrated through protein purification and nitrogen content analysis of extracted purified proteins (Jones, 1931)."
"For many years, the protein content of foods has been determined from total nitrogen content. The protein content in a food is traditionally estimated by multiplying the total nitrogen content by a NPCF, based on the assumptions that dietary carbohydrates and fats do not contain nitrogen, and that most of the nitrogen in the diet is present as amino acids in proteins. However, protein and nitrogen content are defined differently (Jones, 1931; Tkachuk, 1969; Sosulski & Imafidon, 1990; Mossé, 1990; Maubois & Lorient, 2016; Krul, 2019) (Table 1)."
"The conversion factor was historically set at 6.25 by assuming that most, if not all, nitrogen in food was derived from protein, and that the nitrogen content of proteins was about 16%. This approach is still an accepted method for calculating the crude protein content of foods and food ingredients; however, it has been known for decades that using total nitrogen content with a conversion factor of 6.25 to quantify total protein is imperfect and can lead to a 15–20% error in the actual protein content."
"The errors for deriving the crude protein content from nitrogen content have three main origins, as outlined below:
– Total nitrogen content varies between the different amino acids; thus, because amino acid composition varies from one protein source to another, the protein nitrogen content also varies between different protein sources.
– In addition to amino acids, some proteins contain prosthetic groups, some of which are non-nitrogenous and therefore increase the molecular weight of the protein without significantly affecting the nitrogen content. In turn, this results in an increase in the conversion factor, because both amino acids and amino acids plus prosthetic groups are being considered on a weight basis.
– The fraction of non-protein nitrogen varies in different food protein sources; this can result in a lower value of the conversion factor when the total nitrogen content of foods is being considered."
"Newer approaches use values for protein weight and protein nitrogen content of different proteins based on knowledge of the amino acid composition as obtained via amino acid analysis or amino acid sequencing (Heathcote, 1950; Tkachuk, 1969; Holt & Sosulski, 1979; Morr, 1981; Mossé, 1990)." "Calculation of the weight of the protein can include either the sum of the amino acid residues only, or the sum of the amino acid residues plus the weight of the associated prosthetic groups when these are analysed by specific methods or obtained from amino acid sequencing."
"An important feature of approaches based on amino acid composition is the use of the anhydrous weight of amino acids to determine protein weight. Using the free amino acid weight for a protein would grossly overestimate the weight for longer polypeptide chains. Because each amino acid residue in a polypeptide chain loses one water molecule (weighing 18 Da) during polymerization, except for the residue at the carboxy terminus; thus, the weight of the protein must be assessed as the sum of the weight of the anhydrous amino acid residues rather than the weight of the free amino acids (each of which weighs 18 Da more than its anhydrous counterpart)."
"Molecular weights of free and anhydrous forms of all 20 amino acids, and the corresponding percentage of nitrogen, are shown in Table 2."
"Another important consideration when calculating NPCFs relates to the differential contribution to nitrogen content of the amide forms of the amino acids, glutamine and asparagine, and their respective acid forms, glutamic acid and aspartic acid.
With standard amino acid analysis of a protein sample, glutamine and asparagine are converted to glutamic acid and aspartic acid during acid hydrolysis (by substitution of a carboxyl group for the amide group); the total of glutamic acid plus glutamine and aspartic acid plus asparagine is then determined. This conversion does not significantly affect the calculation of the total anhydrous amino acid weight, because there is little difference between the molecular weights of glutamic acid and glutamine (147.13 Da and 146.15 Da, respectively) and aspartic acid and asparagine (133.11 Da and 132.12 Da, respectively). However, failing to consider the ratio between the amide and acid forms induces a major error in the calculation of total nitrogen content of the amino acid residues."
- Converting Nitrogen into Protein—Beyond 6.25 and Jones' Factors
"Different values have been reported by other authors for other proteins, and even for different protein fractions within the same protein source. For example, the nitrogen content of collagen is 18% [reversing it: 100% ÷ 18% = 5.55; was is lower factor, will is nitrogenated]. Consequently, the nitrogen-to-protein ratio in meat products will vary as a function of the collagen level (Benedict, 1987)."
"Interestingly, a major controversy has [..] arisen after it was proposed by the [..] Codex Alimentarius committee guidelines for infant formula standards that, at variance with standard regulations, 6.38 rather than 6.25 should be used for cow milk protein (Koletzko and Shamir, 2006). Indeed, conversion factors have huge financial implications. In the present case, the difference between 6.38 and 6.25 in this regulatory setting would translate into a $96 M difference affecting the European dairy industry (Koletzko and Shamir, 2006)."
"More recent data have provided a clearer understanding of variations in nitrogen-to-protein conversion factors. Genetic variability in foodstuffs and the effects of how protein sources are produced and processed may to a certain extent explain the discrepancies between values determined for the same type of foodstuff. However, the most recent extensive data published by Mossé et al. (1990) and by Sosulski and Imafidon (1990) are sufficiently broad-ranging and methodologically reliable to be considered. Data from Tkachuk (1969) have also been included. Some additional values have been calculated from complete amino acid analyses, including amide nitrogen determinations. Finally, the results obtained by these authors concerning the same plant protein source are often very similar, even if some exceptions exist. The data as a whole are consistent enough to derive a series of nitrogen-to-protein conversion factors that, despite some uncertainties, can be used to estimate more accurately the potential of a protein source to provide nitrogen and amino acids (Table 5)."

"These data also allow us to propose an average default factor (5.60) which is more appropriate than 6.25. A summary of the average factors for the main classes of dietary protein foodstuffs is given in Table 6."

"Interestingly, a major controversy has [..] arisen after it was proposed by the [..] Codex Alimentarius committee guidelines for infant formula standards that, at variance with standard regulations, 6.38 rather than 6.25 should be used for cow milk protein (Koletzko and Shamir, 2006). Indeed, conversion factors have huge financial implications. In the present case, the difference between 6.38 and 6.25 in this regulatory setting would translate into a $96 M difference affecting the European dairy industry (Koletzko and Shamir, 2006)."
"More recent data have provided a clearer understanding of variations in nitrogen-to-protein conversion factors. Genetic variability in foodstuffs and the effects of how protein sources are produced and processed may to a certain extent explain the discrepancies between values determined for the same type of foodstuff. However, the most recent extensive data published by Mossé et al. (1990) and by Sosulski and Imafidon (1990) are sufficiently broad-ranging and methodologically reliable to be considered. Data from Tkachuk (1969) have also been included. Some additional values have been calculated from complete amino acid analyses, including amide nitrogen determinations. Finally, the results obtained by these authors concerning the same plant protein source are often very similar, even if some exceptions exist. The data as a whole are consistent enough to derive a series of nitrogen-to-protein conversion factors that, despite some uncertainties, can be used to estimate more accurately the potential of a protein source to provide nitrogen and amino acids (Table 5)."
"These data also allow us to propose an average default factor (5.60) which is more appropriate than 6.25. A summary of the average factors for the main classes of dietary protein foodstuffs is given in Table 6."
Amazoniac
Member
Amazoniac
Member
Food Sci Nutr. 2018 Jun; 6(4): 1023–1031.
Gelatin is an anti‐inflammatory dietary component, and its predominant metabolites entering circulation are prolyl‐hydroxyproline (Pro‐Hyp) and glycine. We evaluated the protective effects of orally administered gelatin, glycine, and Pro‐Hyp 10:3:0.8 (w/w/w) against dextran sodium sulfate (DSS)‐induced colitis in mice. According to clinical, histological, and biochemical parameters, they exhibited significant activities in the order of gelatin < glycine < Pro‐Hyp. Gelatin prevented the DSS‐induced increase in interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6), and tumor necrosis factor‐α (TNF‐α) in the colon, rather than in peripheral blood. Glycine and Pro‐Hyp attenuated the DSS‐induced rise in colonic IL‐6 and TNF‐α, as well as peripheral IL‐1β, IL‐6, and TNF‐α. Hematologic results show the attenuation of DSS‐induced leukocytosis and lymphocytosis by glycine and Pro‐Hyp, rather than gelatin. These findings suggest that glycine and Pro‐Hyp constitute the material basis for gelatin's anticolitis efficacy, and they have better anticolitis activities and distinct mechanisms of action when ingested as free compounds than as part of gelatin.
"Although gelatin has a digestibility as high as 98.8% (Reuterswärd & Fabiansson, 2010), it displayed a significantly lower anticolitis activity than glycine and Pro‐Hyp according to DAI, colon lengths, colon histology, and biochemical parameters (Figures 1 and and2).2). This is probably because gelatin is not completely digested and absorbed in the small intestine (Chen, Rogers, & Harper, 1962; Nixon & Mawer, 1970)."
"Orally administered glycine and Pro‐Hyp, which can be very efficiently absorbed in the small intestine (Craft, Geddes, Hyde, Wise, & Matthews, 1968; Yamamoto et al., 2015), should therefore induce higher peak concentrations of glycine and Pro‐Hyp in circulating blood than gelatin, and this may explain their superior anticolitis activities."
"Oral gelatin was ineffective in attenuating the increase in serum cytokines (Figure 4), but prevented the colitis‐associated anemia effectively. It thus seems that the antianemia activity of oral gelatin during IBD was independent on its anti‐inflammatory activity."
"Glycine and Pro‐Hyp have higher anticolitis activities when ingested as free compounds than as part of gelatin. Gelatin's anticolitis activity possibly depends on the anti‐inflammatory effects of glycine and Pro‐Hyp in situ absorbed by the colon. Oral glycine and Pro‐Hyp perform their anticolitis activities probably by means of circulating glycine and Pro‐Hyp, which inhibit peripheral cytokine production thereby preventing leukocyte mobilization into the circulation."
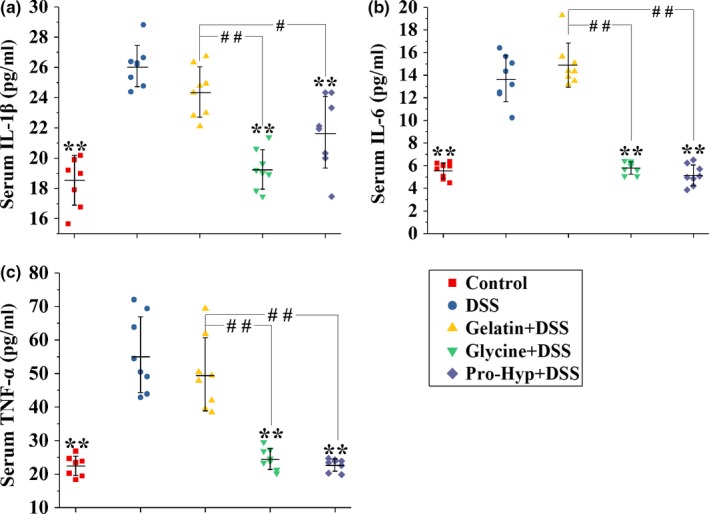
Gelatin is an anti‐inflammatory dietary component, and its predominant metabolites entering circulation are prolyl‐hydroxyproline (Pro‐Hyp) and glycine. We evaluated the protective effects of orally administered gelatin, glycine, and Pro‐Hyp 10:3:0.8 (w/w/w) against dextran sodium sulfate (DSS)‐induced colitis in mice. According to clinical, histological, and biochemical parameters, they exhibited significant activities in the order of gelatin < glycine < Pro‐Hyp. Gelatin prevented the DSS‐induced increase in interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6), and tumor necrosis factor‐α (TNF‐α) in the colon, rather than in peripheral blood. Glycine and Pro‐Hyp attenuated the DSS‐induced rise in colonic IL‐6 and TNF‐α, as well as peripheral IL‐1β, IL‐6, and TNF‐α. Hematologic results show the attenuation of DSS‐induced leukocytosis and lymphocytosis by glycine and Pro‐Hyp, rather than gelatin. These findings suggest that glycine and Pro‐Hyp constitute the material basis for gelatin's anticolitis efficacy, and they have better anticolitis activities and distinct mechanisms of action when ingested as free compounds than as part of gelatin.
"Although gelatin has a digestibility as high as 98.8% (Reuterswärd & Fabiansson, 2010), it displayed a significantly lower anticolitis activity than glycine and Pro‐Hyp according to DAI, colon lengths, colon histology, and biochemical parameters (Figures 1 and and2).2). This is probably because gelatin is not completely digested and absorbed in the small intestine (Chen, Rogers, & Harper, 1962; Nixon & Mawer, 1970)."
"Orally administered glycine and Pro‐Hyp, which can be very efficiently absorbed in the small intestine (Craft, Geddes, Hyde, Wise, & Matthews, 1968; Yamamoto et al., 2015), should therefore induce higher peak concentrations of glycine and Pro‐Hyp in circulating blood than gelatin, and this may explain their superior anticolitis activities."
"Oral gelatin was ineffective in attenuating the increase in serum cytokines (Figure 4), but prevented the colitis‐associated anemia effectively. It thus seems that the antianemia activity of oral gelatin during IBD was independent on its anti‐inflammatory activity."
"Glycine and Pro‐Hyp have higher anticolitis activities when ingested as free compounds than as part of gelatin. Gelatin's anticolitis activity possibly depends on the anti‐inflammatory effects of glycine and Pro‐Hyp in situ absorbed by the colon. Oral glycine and Pro‐Hyp perform their anticolitis activities probably by means of circulating glycine and Pro‐Hyp, which inhibit peripheral cytokine production thereby preventing leukocyte mobilization into the circulation."

Amazoniac
Member
Amazoniac
Member
D
Deleted member 5487
Guest
Favorite Supplement right now. Take 20 a day. Don't even like eating protein now lol, seems more like a cultural chore.

I add glycine and proline with it, 1g each.
I add glycine and proline with it, 1g each.
looking2grow96
Member
- Joined
- Feb 7, 2020
- Messages
- 215
Sorry, you take 20 what a day?Favorite Supplement right now. Take 20 a day. Don't even like eating protein now lol, seems more like a cultural chore.
View attachment 34171
I add glycine and proline with it, 1g each.
Bootselectric
Member
- Joined
- Apr 9, 2018
- Messages
- 82
@Amarsh213 Curious about this as well.
And is your diet still fruit and meat? Do you take amino acids?
And is your diet still fruit and meat? Do you take amino acids?
D
Deleted member 5487
Guest
I eat everything to be honest. I had butter, eggs, potatos and ammino acids for breakfast/lunch and have a big fatty chuck roast, potato, onion in the crock pot right now.@Amarsh213 Curious about this as well.
And is your diet still fruit and meat? Do you take amino acids?
Im still working out my diet and learning.
But I do know 1 thing for sure is adding some Betaline-HCL, digestive enzymes, and ammino acids( MAAP) drastically increased my health. Doesn't matter how much protein we eat if you cannot digest it.
Most people probably have systemic protein deficiencies and lack the HCL to digest what they do eat when they try to correct it.
@haidut says in this post that ammino acids might be needed to help jump start. I really don't think most people here even digest their protein to any degree.
I can't even imagine eating a steak without a few Betaline-HCL tablet before.
D
Deleted member 5487
Guest
Those 1 gram MAAP that haidut talks about here. Im chewing up about 15-20 a day. Expensive but it seems to work very well. Much easier than trying to digest protein rn.Sorry, you take 20 what a day?
Amazon product ASIN B0081KWNHYView: https://www.amazon.com/Master-Amino-Acid-Pattern-MAP/dp/B0081KWNHY/ref=sr_1_6?crid=1N6K9329EJQ5&keywords=master+amino+acid+pattern+map&qid=1646606209&sprefix=master+ammino%2Caps%2C393&sr=8-6
Read the reviews. Pretty much spot on as others have said. It seems to cure systemic protein deficiencies that about 90% of people probably have unleashes all dominos effects.
Bootselectric
Member
- Joined
- Apr 9, 2018
- Messages
- 82
Got it. So, not much milk / cheese, I suppose.I eat everything to be honest. I had butter, eggs, potatos and ammino acids for breakfast/lunch and have a big fatty chuck roast, potato, onion in the crock pot right now.
Im still working out my diet and learning.
But I do know 1 thing for sure is adding some Betaline-HCL, digestive enzymes, and ammino acids( MAAP) drastically increased my health. Doesn't matter how much protein we eat if you cannot digest it.
Most people probably have systemic protein deficiencies and lack the HCL to digest what they do eat when they try to correct it.
@haidut says in this post that ammino acids might be needed to help jump start. I really don't think most people here even digest their protein to any degree.
I can't even imagine eating a steak without a few Betaline-HCL tablet before.
How about salt… do you still salt carefully (if at all)?
I am going to try the aminos and betain hcl. :)
Amazoniac
Member
- Environmental enteropathy: new targets for nutritional interventions
"Only an optimally functioning gut can utilise all of the nutrients from the diet and form an effective barrier to protect the individual from hostile attacks. Since the gut tissue itself will consume all of the dietary nutrients that it requires before allowing the passage of excess nutrients to the rest of the body for routine maintenance and growth[50] we hypothesise that nutritional interventions aimed at improving/repairing gut structure and function will ultimately improve the growth of children with EE. For example, by supplying the optimal nutrient profile required by the inflamed and injured gut (e.g. specific amino acids such as glutamine, threonine, leucine and cysteine) as building blocks for mucin production,[51,52] repair or for conversion to other useful amino acids and as a source of energy.[53]"
I am trying this at the moment, but is no one worried about BCAAs causing obesity?
For example:

 pubmed.ncbi.nlm.nih.gov
pubmed.ncbi.nlm.nih.gov

 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
Methionine restriction is supposed to aid fat loss, but if you need BCAA's to restrict methionine, I don't understand how it all works out...
For example:
Key points: We recently found that feeding healthy mice a diet with reduced levels of branched-chain amino acids (BCAAs), which are associated with insulin resistance in both humans and rodents, modestly improves glucose tolerance and slows fat mass gain. In the present study, we show that a reduced BCAA diet promotes rapid fat mass loss without calorie restriction in obese mice. Selective reduction of dietary BCAAs also restores glucose tolerance and insulin sensitivity to obese mice, even as they continue to consume a high-fat, high-sugar diet. A low BCAA diet transiently induces FGF21 (fibroblast growth factor 21) and increases energy expenditure. We suggest that dietary protein quality (i.e. the precise macronutrient composition of dietary protein) may impact the effectiveness of weight loss diets.

Restoration of metabolic health by decreased consumption of branched-chain amino acids - PubMed
Obesity and diabetes are increasing problems around the world, and although even moderate weight loss can improve metabolic health, reduced calorie diets are notoriously difficult to sustain. Branched-chain amino acids (BCAAs; leucine, isoleucine and valine) are elevated in the blood of obese...
Branched-chain amino acids (BCAAs) are essential amino acids that are not synthesized in our body; thus, they need to be obtained from food. They have shown to provide many physiological and metabolic benefits such as stimulation of pancreatic insulin secretion, milk production, adipogenesis, and enhanced immune function, among others, mainly mediated by mammalian target of rapamycin (mTOR) signaling pathway. After identified as a reliable marker of obesity and type 2 diabetes in recent years, an increasing number of studies have surfaced implicating BCAAs in the pathophysiology of other diseases such as cancers, cardiovascular diseases, and even neurodegenerative disorders like Alzheimer's disease. Here we discuss the most recent progress and review studies highlighting both correlational and potentially causative role of BCAAs in the development of these disorders. Although we are just beginning to understand the intricate relationships between BCAAs and some of the most prevalent chronic diseases, current findings raise a possibility that they are linked by a similar putative mechanism.

Recent Progress on Branched-Chain Amino Acids in Obesity, Diabetes, and Beyond
Branched-chain amino acids (BCAAs) are essential amino acids that are not synthesized in our body; thus, they need to be obtained from food. They have shown to provide many physiological and metabolic benefits such as stimulation of pancreatic insulin ...
Methionine restriction is supposed to aid fat loss, but if you need BCAA's to restrict methionine, I don't understand how it all works out...
This study has a few interesting explanations:

 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
However,
That is, mice that got more BCAAs were more hungry and ate more, which made them fat and caused them to die earlier. When they were given the same amount of food as the control mice, they didn't get fat and lived equally long.
We want to avoid tryptophan, but BCAAs + threonine also did not lead to overeating and obesity. So I guess BCAA + threonine + lysine (+ taurine + beta-alanine + glycine optionally) is still alright.

Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control
Elevated branched chain amino acids (BCAAs) are associated with obesity and insulin resistance. How long-term dietary BCAAs impact late-life health and lifespan is unknown. Here, we show that when dietary BCAAs are varied against a fixed, isocaloric macronutrient ...
Here, we show that when dietary BCAAs are varied against a fixed, isocaloric macronutrient background, long-term exposure to high BCAA diets leads to hyperphagia, obesity and reduced lifespan
However,
We discovered that the metabolic burden of dietary BCAAs under high carbohydrate intakes was not due to increased hepatic mTOR activation, but primarily driven by hyperphagia.
That is, mice that got more BCAAs were more hungry and ate more, which made them fat and caused them to die earlier. When they were given the same amount of food as the control mice, they didn't get fat and lived equally long.
Supplementing only the metabolically essential AAs Trp or Thr, thereby rebalancing the diet for these AAs against BCAAs, significantly reduced hyperphagia associated with the BCAA200 diet.
We want to avoid tryptophan, but BCAAs + threonine also did not lead to overeating and obesity. So I guess BCAA + threonine + lysine (+ taurine + beta-alanine + glycine optionally) is still alright.
Lejeboca
Member
- Joined
- Jun 19, 2017
- Messages
- 1,039
Feeding intact proteins, peptides, or free amino acids to monogastric farm animals
For terrestrial farm animals, intact protein sources like soybean meal have been the main ingredients providing the required amino acids (AA) to sustain life. However, in recent years, the availability of hydrolysed protein sources and free AA has led to the use of other forms of AA to feed farm animals. The advent of using these new forms is especially important to reduce the negative environmental impacts of animal production because these new forms allow reducing the dietary crude protein content and provide more digestible materials. However, the form in which dietary AA are provided can have an effect on the dynamics of nutrient availability for protein deposition and tissue growth including the efficiency of nutrient utilization. In this literature review, the use of different forms of AA in animal diets is explored, and their differences in digestion and absorption rates are focused on. These differences affect the postprandial plasma appearance of AA, which can have metabolic consequences, like greater insulin response when free AA or hydrolysates instead of intact proteins are fed, which can have a profound effect on metabolism and growth performance. Nevertheless, the use and application of the different AA forms in animal diets are important to achieve a more sustainable and efficient animal production system in the future, as they allow for a more precise diet formulation and reduced negative environmental impact. It is, therefore, important to differentiate the physiological and metabolic effects of different forms of AA to maximize their nutritional value in animal diets.
The Optimum Form of Dietary Nitrogen in Gastrointestinal Disease: Proteins, Peptides or Amino Acids?
At one time it was thought that proteins had to be hydrolysed to free amino acids before absorption but this hypothesis was finally overturned in the 1960’s and it is now clear that peptide absorption in the gastrointestinal tract is of major nutritional significance. The products of luminal hydrolysis (free amino acids and small peptides) can be absorbed either as free amino acids—by four group specific amino acid transport systems, or as intact di— and tripeptides—by a separate transport system. Tetra— and higher peptides require brush border hydrolysis and the products are absorbed either as free amino acids or as di— and tripeptides.
EMF Mitigation - Flush Niacin - Big 5 Minerals
Similar threads
- Replies
- 7
- Views
- 6K
- Replies
- 12
- Views
- 5K
- Replies
- 1
- Views
- 4K
- Replies
- 0
- Views
- 2K
- Replies
- 5
- Views
- 3K
- Replies
- 3
- Views
- 4K
- Replies
- 43
- Views
- 20K
- Replies
- 6
- Views
- 3K
- Replies
- 22
- Views
- 17K
