Inaut
Member
- Joined
- Nov 29, 2017
- Messages
- 3,620
In this interview, Dr. Hamblin explains:
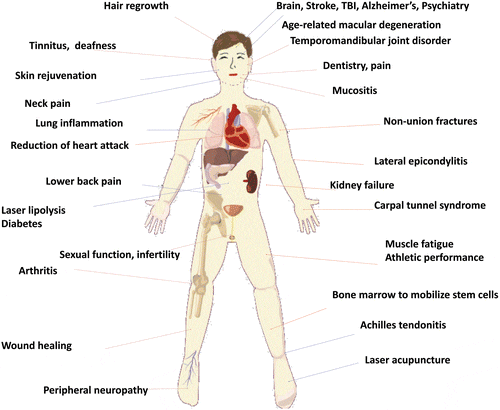
What photobiomodulation is, and the molecular mechanisms through which it works its magic
What wavelengths and intensities of light are used for physiological effects
How photobiomodulation has been investigated for athletic performance, skin health and rejuvenation, and psychological conditions
When and how to use red light therapy for exercise performance and recovery
How red light functions as a healthy stressor to elicit anti-aging effects