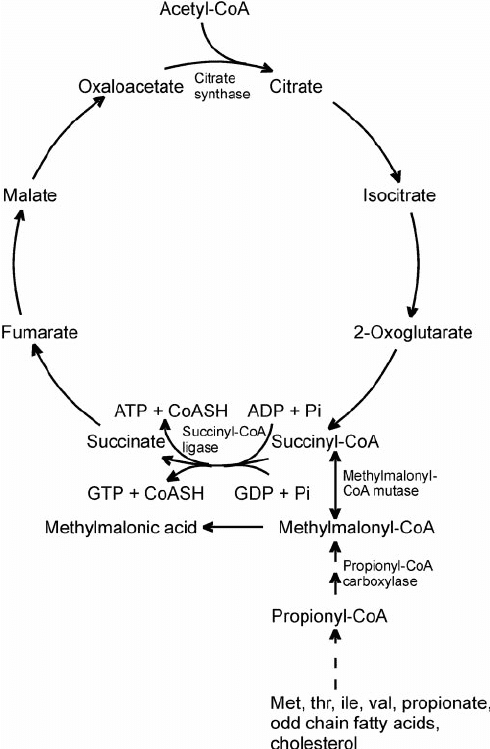
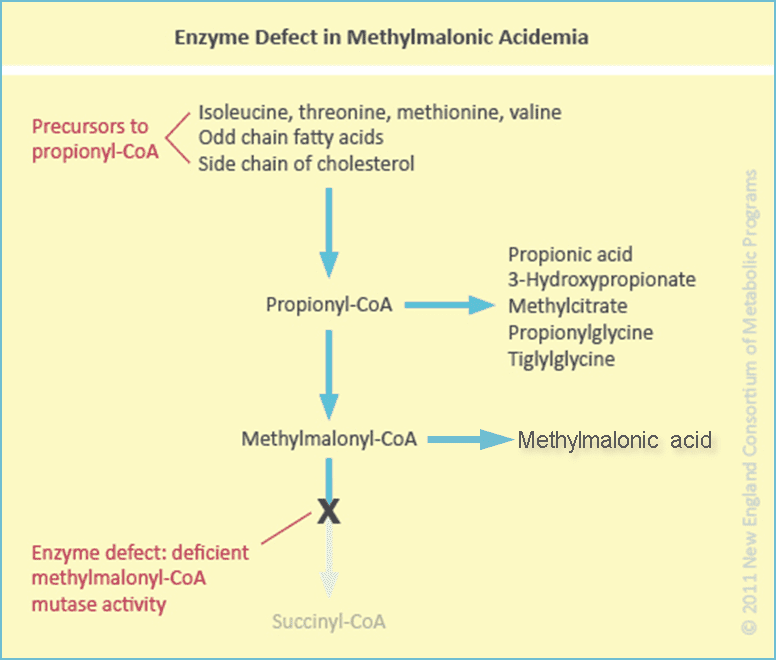
The above was copied from Amazoniac's further above. It could apply to me. I probably have impaired intestinal function. I'm gluten intolerant. This impaired intestinal function is the reason I do better with injections of B12.
Then, I've dealt with parasitic infections in the past and have some indications of present problems there. I see a connection to my Seborrheic dermatitis and rosacea. To help, I took a weight appropriate dose of Ivermectin a few days ago. Wonder what Peat might think of that...
I will avoid going under with nitrous oxide.
Then, I've dealt with parasitic infections in the past and have some indications of present problems there. I see a connection to my Seborrheic dermatitis and rosacea. To help, I took a weight appropriate dose of Ivermectin a few days ago. Wonder what Peat might think of that...
I will avoid going under with nitrous oxide.