Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
-
By using this site you agree to the terms, rules, and privacy policy.
-
Charlie's Restoration Giveaway #2 (Entire Home EMF Mitigation & Protection Along With Personal Protection) - Click Here To Enter
-
Dear Carnivore Dieters, A Muscle Meat Only Diet is Extremely Healing Because it is a Low "vitamin A" Diet. This is Why it Works so Well...
Rest the rest of this post by clicking here
-
The Forum is transitioning to a subscription-based membership model - Click Here To Read
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Niacinamide Or Just Plain Niacin?
- Thread starter DMF
- Start date
natedawggh
Member
- Joined
- Aug 24, 2013
- Messages
- 649
While both are considered B3, they are not the same. Niacin will give you that flushing sensation, while Niacinamide will not. Also, Niacin can be hard on the liver at high doses, while Niacinamide is not at all and can be used therapeutically at doses that would totally make you feel terrible if you use niacin.
kiran
Member
- Joined
- Aug 9, 2012
- Messages
- 1,054
Niacin is different from Niacinamide, the flush isn't beneficial.
I think you mean glycine. Its found in milk but you might consider gelatin or glycine powder.
I think you mean glycine. Its found in milk but you might consider gelatin or glycine powder.
uuy8778yyi
Member
- Joined
- Dec 21, 2014
- Messages
- 289
the no flush niacin, is toxic if you read internet sources
you want the flush
you want the flush
Such_Saturation
Member
- Joined
- Nov 26, 2013
- Messages
- 7,370
Niacin liberates PUFA in your blood.
Parsifal
Member
- Joined
- Aug 6, 2015
- Messages
- 1,081
So Niacinamide can help to detox PUFAs from your body?Such_Saturation said:https://raypeatforum.com/forums/posts/89862/ Niacin liberates PUFA in your blood.
Last edited by a moderator:
Such_Saturation
Member
- Joined
- Nov 26, 2013
- Messages
- 7,370
Parsifal said:https://raypeatforum.com/forums/posts/97687/So Niacinamide can help to detox PUFAs from your body?Such_Saturation said:https://raypeatforum.com/forums/posts/89862/ Niacin liberates PUFA in your blood.
I've been wondering that! But Ray Peat would probably say it's safer to detoxify them from inside your fat stores, more slowly.
Last edited by a moderator:
Parsifal
Member
- Joined
- Aug 6, 2015
- Messages
- 1,081
That's interesting, I've seen a video some times ago and the guy say that niacine is used to detox agent orange: https://www.youtube.com/watch?v=47UyvsCQ-mk
Such_Saturation
Member
- Joined
- Nov 26, 2013
- Messages
- 7,370
If you give niacin to a schizophrenic patient he won't flush  all his PUFA already exploded in his body.
all his PUFA already exploded in his body.
 all his PUFA already exploded in his body.
all his PUFA already exploded in his body.Parsifal
Member
- Joined
- Aug 6, 2015
- Messages
- 1,081
Does niacin promotes histamines creation or is it an histamine detoxifier?
I know, Peat recommends niacinamide and not nicotinic acid, because nicotinic acid tends to increase prostaglandins (and causing the so-called "niacin flush"). Nicotinic acid also seems to decrease cholesterol in high doses, which is probably bad, as it could indicate some liver damage. However, I have seen some studies pointing out beneficial effects with nicotinic acid too, for example, there were studies showing improved memory. And interestingly, Dr. Max Gerson, who cured terminal cancer patients with coffee enemas, juices and a fat-free diet, also had patients take 50 mg of niacin (not niacinamide) several times per day, sometimes his protocol recommends a dose of niacin every hour.
Given these things, wouldn't it be OK to use nicotinic acid form of niacin, if niacinamide is allergenic and causes bad reactions?
Have you tried nicotinic acid and what were the benefits or side-effects?
Given these things, wouldn't it be OK to use nicotinic acid form of niacin, if niacinamide is allergenic and causes bad reactions?
Have you tried nicotinic acid and what were the benefits or side-effects?
Amazoniac
Member
@Wiscounselor - do you have an opinion on this topic?
Niacin sometimes refers to nicotinic acid or... | Ray Peat Forum
Niacin sometimes refers to nicotinic acid or... | Ray Peat Forum
Travis
Member
- Joined
- Jul 14, 2016
- Messages
- 3,189
Yes. I was just thinking about this a few minutes ago when I was replying to a niacin‐related post. After searching historical articles, It appears as though 'niacin' has always been used to denote nicotinic acid. You'd think the '–in' suffix would have been originally added to the amine form, but if so it wasn't used like that for long. Perhaps this suffix '–in' had been derived from the word 'vitamin?'@Wiscounselor - do you have an opinion on this topic?
Niacin sometimes refers to nicotinic acid or... | Ray Peat Forum
Last edited:
Frankdee20
Member
I read that niacin uses up histamine by releasing it, over time, lowers it
Amazoniac
Member
From now on, in your honor I'll make the distinction: nicotinic acid and niacinamide/nicotinamide.Yes. I was just thinking about this a few minutes ago when I was replying to a niacin‐related post. After searching historical articles, It appears as though 'niacin' has always been used to denote nicotinic acid. You'd think the '–in' suffix would have been originally added to the amine form, but if so it wasn't used like that for long. Perhaps this suffix '–in' had been derived from the word 'vitamin?'
- Metabolic Response of Humans to Ingestion of Nicotinic Acid and Nicotinamide
- On the Relative Efficacy of Nicotinamide and Nicotinic Acid as Precursors of Nicotinamide Adenine Dinucleotide (rats, injected)
Amazoniac
Member
The first bite on an apfel doesn't leave you with pieces of the skin between teeth. It's not as common to happen during chewing as it is when taking the following bites. That's because apfels are smaller than the length of two mouths, therefore part of the next bite is going to coincide with the previous. In doing so, it's common to place the skin between teeth and pieces of it get trapped just to annoy you. If you bite it in a way that the skin is positioned in the middle of the tooth, this can be avoided.
But this has nothing to do with the fact that it's very unlikely that nicotinic acid in low doses is toxic. This is one more case in which Gerson's approach deserves to be mentioned. The guy spent a great deal of his book talking about the importance of the liver, how most of his debilitated patients had it impaired and how careful you have to be to avoid overwhelming it. Nicotinamide was right there waving at him to be picked, but he refused and there must be a good reason why.
The previous paragraph ended up being a bit longer than the first, which reinforces that I'm giving more importance to the relevant subject.
I think I'm learning Travis' humor as the reader can tell by examining the previous sentence but also the current one.
Oh crαp, I had to edit the first due to mistakes, so that doesn't apply anymore.
- GI Absorption of Niacin in Humans
- NAD+ and Vitamin B3: From Metabolism to Therapies
- Safety of high-dose nicotinamide: a review
- Metabolism of Nicotinic Acid and Related Compounds in Man and Rat
- Nicotinamide supplementation induces detrimental metabolic and epigenetic changes in developing rats
- Nicotinamide N-Methyltransferase: More Than a Vitamin B3 Clearance Enzyme
- The Metabolism of Radioactive Nicotinic Acid and Nicotinamide
- https://raypeatforum.com/community/threads/incomprehensive-ble-notes-on-choline.23228/#post-329705
But this has nothing to do with the fact that it's very unlikely that nicotinic acid in low doses is toxic. This is one more case in which Gerson's approach deserves to be mentioned. The guy spent a great deal of his book talking about the importance of the liver, how most of his debilitated patients had it impaired and how careful you have to be to avoid overwhelming it. Nicotinamide was right there waving at him to be picked, but he refused and there must be a good reason why.
The previous paragraph ended up being a bit longer than the first, which reinforces that I'm giving more importance to the relevant subject.
I think I'm learning Travis' humor as the reader can tell by examining the previous sentence but also the current one.
Oh crαp, I had to edit the first due to mistakes, so that doesn't apply anymore.
- GI Absorption of Niacin in Humans
I guess nicotinic acid was used but their absorbed amount doesn't differ.
"In a steady-state situation, about 85% of an orally administered dose (3 g/day) of niacin was recovered from the urine, and it was concluded that absorption was nearly complete (3)."
"The highest level of niacin in plasma was found 5-10 min after instillation [200 mg, tube] into the upper small intestine[.] During instillation into the stomach [same], the maximum plasma niacin concentration occurred 10-20 min after drug administration. Evidently, food did not influence niacin absorption, as evidenced by the peak plasma concentrations with and without a standard meal[.]"
"After instillation into the small intestine of an aqueous niacin solution, the drug appears faster in the plasma and reaches higher levels than after instillation into the stomach. This finding indicates that the compound is absorbed at least as readily from the small intestine as from the stomach."
"In a steady-state situation, about 85% of an orally administered dose (3 g/day) of niacin was recovered from the urine, and it was concluded that absorption was nearly complete (3)."
"The highest level of niacin in plasma was found 5-10 min after instillation [200 mg, tube] into the upper small intestine[.] During instillation into the stomach [same], the maximum plasma niacin concentration occurred 10-20 min after drug administration. Evidently, food did not influence niacin absorption, as evidenced by the peak plasma concentrations with and without a standard meal[.]"
"After instillation into the small intestine of an aqueous niacin solution, the drug appears faster in the plasma and reaches higher levels than after instillation into the stomach. This finding indicates that the compound is absorbed at least as readily from the small intestine as from the stomach."
- NAD+ and Vitamin B3: From Metabolism to Therapies
"High doses of nicotinamide administered orally or through injection are transiently metabolized in liver to increase NAD+. However, nicotinamide at elevated doses can cause hepatotoxicity. Nicotinamide is methylated to form 1-methylnicotinamide and downstream oxidized pyridones as metabolic end products (Knip et al., 2000 [below]). Large doses of nicotinamide cause methyl donor depletion (Knip et al., 2000). A large portion of nicotinamide administered to rats at 500 mg/kg was excreted unchanged within 12 h after injection. The remainder of nicotinamide was generally excreted as methylated or oxidized forms of the pyridine (Knip et al., 2000). At non-pharmacologic doses, nicotinamide is lost, mostly by excretion of the catabolic products, rather than as the unmetabolized vitamin."
"Nicotinic acid is rapidly metabolized by the liver and can be catabolized by glycine conjugation to nicotinuric acid (Capuzzi et al., 2000). Nicotinic acid increases NAD+ content in liver but is generally no more effective than nicotinamide in this respect (Jackson et al., 1995), indicating that NAD+ biosynthesis in liver is not a likely explanation for nicotinic acid correction effects in hyperlipidemia."
"Nicotinic acid is rapidly metabolized by the liver and can be catabolized by glycine conjugation to nicotinuric acid (Capuzzi et al., 2000). Nicotinic acid increases NAD+ content in liver but is generally no more effective than nicotinamide in this respect (Jackson et al., 1995), indicating that NAD+ biosynthesis in liver is not a likely explanation for nicotinic acid correction effects in hyperlipidemia."
- Safety of high-dose nicotinamide: a review
"Nicotinamide is readily absorbed parenterally and from all parts of the gastrointestinal tract [4] and peak concentrations are achieved in humans within about 1 hour of oral ingestion of standard preparations [27]. Nicotinamide disappears rapidly from the circulation and is distributed in all tissues. It has a high hepatic extraction ratio and plasma clearance can be reduced in patients with hepatic insufficiency."
"Nicotinamide can be oxidised to nicotinamide-N-oxide, methylated to N-methyl-nicotinamide or hydroxylated to 6-hydroxynicotinamide (Fig. 1)."
"Hepatic methylation using l-methionine as a methyl donor is important in the detoxification of nicotinamide [23]. The product of this reaction, N-methyl-nicotinamide, is excreted by the kidneys whereas nicotinamide is reabsorbed by the renal tubules [29]. For this reason only small amounts of the unchanged vitamin appear in the urine even at pharmacological doses of nicotinamide. N-methyl nicotinamide is oxidised in the liver, a process that is saturated at high circulating concentrations, and the end products are N-methyl-2-pyridone-5-carboxylic acid amide 2 pyr) and N-methyl-4-pyridone-3-carboxylic acid amide 4 pyr). The ratio of the two metabolites differs with species and sex. In humans 2 pyr is formed exclusively [28]. Nicotinamide oxidation to nicotinamide-N-oxide can also be [do not confuse with Bee, Jennifer's dog] an important pathway at very high doses in humans and other species [23, 30]."
"Older clinical studies using nicotinic acid or impure preparations of nicotinamide reported relatively frequent liver enzyme abnormalities [6, 33±35] although more recent studies using a purified form of nicotinamide have not detected any noteworthy adverse effects on liver enzymes [13, 15, 17, 20, 36]."
"The oxidation of ethanol leads to an increase in the NADH:NAD ratio which secondarily decreases the activity of key enzymes on ATP-producing pathways and so decreases the production of albumin and fibrinogen by the liver. When nicotinamide is given with ethanol and a standard meal, it counteracts this effect and so restores the meal-induced increase in albumin and fibrinogen."
"nicotinic acid increases uric acid concentrations in some subjects"
"Because nicotinamide potentially promotes survival of cells with DNA damage, possible oncogenicity is an issue of concern, particularly when long-term use of the drug is considered in children and adolescents. Nicotinamide does not seem to have any oncogenic effect when given alone." "Nicotinamide can however potentiate the ability of streptozotozin or alloxan to induce pancreatic islet cell tumours in rodents [46, 48-51]. The dose of nicotinamide used in these experiments was high, ranging from 305 to 500 mg/kg. There has been no suggestion of oncogenic effects in humans despite high-dose treatment of more than 2000 people in studies dating back over many years [5±20, 33, 35, 52±57]. The quality and completeness of follow-up in these reports is however variable. It is therefore most important that future studies of nicotinamide in prediabetes where DNA damage has already occurred include careful long-term follow-up for evidence of oncogenicity."
"Growth inhibition in rats was first shown in 1942, after inclusion of 1% nicotinamide in the diet. This effect, which was not seen with nicotinic acid, was completely reversed by inclusion of methionine in the diet [25]. No effect on growth was found in young rabbits and guinea-pigs [26]. Inhibition of growth in rats might be due to increased synthesis of N-methyl nicotinamide, resulting in a methionine and hence choline deficiency; the effects of paracetamol upon growth in rodents have a similar basis [58]. If so the species difference is easily explained, as methylation to N-methyl nicotinamide is not the major route of metabolism in the rabbit or guinea-pig. The unpleasant taste of nicotinamide in solution might explain why food intake was reduced by almost half in nicotinamide-treated animals in one study [25]."
"A low methionine [unlike choline?] diet does not affect hepatic methylation in humans [59] and nicotinamide has not been shown to affect growth in children. When height and weight were monitored in 173 children under the age of 12 who were positive for islet cell antibodies (ICA) and were treated with nicotinamide (1 g daily) and for whom two or more readings for height and weight were available, a regression across time showed no change in standard deviation units of height, suggesting that linear growth was not affected [19]."
"Glucose kinetics or basal or stimulated insulin concentrations are unaffected in healthy subjects [61, 62]. In contrast, nicotinic acid can induce insulin resistance and glucose intolerance [40]"
"In patients with recently diagnosed diabetes a meta-analysis of 10 randomised controlled trials found that basal C-peptide concentrations were higher in patients receiving nicotinamide than in those receiving placebo 12 months from diagnosis [63]. Published studies of nicotinamide treatment in people at increased risk of developing diabetes have produced varying results. In one small study insulin sensitivity decreased after two weeks of nicotinamide, although basal and stimulated insulin secretion were unchanged [64], whereas DENIS found a decreased first-phase insulin response in nicotinamide-treated people at 2 years [20]."
"Higher doses of nicotinamide should still be considered as having toxic potential."
"Nicotinamide can be oxidised to nicotinamide-N-oxide, methylated to N-methyl-nicotinamide or hydroxylated to 6-hydroxynicotinamide (Fig. 1)."
"Hepatic methylation using l-methionine as a methyl donor is important in the detoxification of nicotinamide [23]. The product of this reaction, N-methyl-nicotinamide, is excreted by the kidneys whereas nicotinamide is reabsorbed by the renal tubules [29]. For this reason only small amounts of the unchanged vitamin appear in the urine even at pharmacological doses of nicotinamide. N-methyl nicotinamide is oxidised in the liver, a process that is saturated at high circulating concentrations, and the end products are N-methyl-2-pyridone-5-carboxylic acid amide 2 pyr) and N-methyl-4-pyridone-3-carboxylic acid amide 4 pyr). The ratio of the two metabolites differs with species and sex. In humans 2 pyr is formed exclusively [28]. Nicotinamide oxidation to nicotinamide-N-oxide can also be [do not confuse with Bee, Jennifer's dog] an important pathway at very high doses in humans and other species [23, 30]."
"Older clinical studies using nicotinic acid or impure preparations of nicotinamide reported relatively frequent liver enzyme abnormalities [6, 33±35] although more recent studies using a purified form of nicotinamide have not detected any noteworthy adverse effects on liver enzymes [13, 15, 17, 20, 36]."
"The oxidation of ethanol leads to an increase in the NADH:NAD ratio which secondarily decreases the activity of key enzymes on ATP-producing pathways and so decreases the production of albumin and fibrinogen by the liver. When nicotinamide is given with ethanol and a standard meal, it counteracts this effect and so restores the meal-induced increase in albumin and fibrinogen."
"nicotinic acid increases uric acid concentrations in some subjects"
"Because nicotinamide potentially promotes survival of cells with DNA damage, possible oncogenicity is an issue of concern, particularly when long-term use of the drug is considered in children and adolescents. Nicotinamide does not seem to have any oncogenic effect when given alone." "Nicotinamide can however potentiate the ability of streptozotozin or alloxan to induce pancreatic islet cell tumours in rodents [46, 48-51]. The dose of nicotinamide used in these experiments was high, ranging from 305 to 500 mg/kg. There has been no suggestion of oncogenic effects in humans despite high-dose treatment of more than 2000 people in studies dating back over many years [5±20, 33, 35, 52±57]. The quality and completeness of follow-up in these reports is however variable. It is therefore most important that future studies of nicotinamide in prediabetes where DNA damage has already occurred include careful long-term follow-up for evidence of oncogenicity."
"Growth inhibition in rats was first shown in 1942, after inclusion of 1% nicotinamide in the diet. This effect, which was not seen with nicotinic acid, was completely reversed by inclusion of methionine in the diet [25]. No effect on growth was found in young rabbits and guinea-pigs [26]. Inhibition of growth in rats might be due to increased synthesis of N-methyl nicotinamide, resulting in a methionine and hence choline deficiency; the effects of paracetamol upon growth in rodents have a similar basis [58]. If so the species difference is easily explained, as methylation to N-methyl nicotinamide is not the major route of metabolism in the rabbit or guinea-pig. The unpleasant taste of nicotinamide in solution might explain why food intake was reduced by almost half in nicotinamide-treated animals in one study [25]."
"A low methionine [unlike choline?] diet does not affect hepatic methylation in humans [59] and nicotinamide has not been shown to affect growth in children. When height and weight were monitored in 173 children under the age of 12 who were positive for islet cell antibodies (ICA) and were treated with nicotinamide (1 g daily) and for whom two or more readings for height and weight were available, a regression across time showed no change in standard deviation units of height, suggesting that linear growth was not affected [19]."
"Glucose kinetics or basal or stimulated insulin concentrations are unaffected in healthy subjects [61, 62]. In contrast, nicotinic acid can induce insulin resistance and glucose intolerance [40]"
"In patients with recently diagnosed diabetes a meta-analysis of 10 randomised controlled trials found that basal C-peptide concentrations were higher in patients receiving nicotinamide than in those receiving placebo 12 months from diagnosis [63]. Published studies of nicotinamide treatment in people at increased risk of developing diabetes have produced varying results. In one small study insulin sensitivity decreased after two weeks of nicotinamide, although basal and stimulated insulin secretion were unchanged [64], whereas DENIS found a decreased first-phase insulin response in nicotinamide-treated people at 2 years [20]."
"Higher doses of nicotinamide should still be considered as having toxic potential."
- Metabolism of Nicotinic Acid and Related Compounds in Man and Rat
"The ingestion of nicotinic acid by man is followed within the first 3 hr. by the excretion of a glycine conjugate, nicotinuric acid, and of the NL-methylated nicotinyl derivatives, of which N'-methylnicotinamide could be detected in these studies. It is interesting to find a small rise in the excretion of nicotinamide, indicating not only that amidation has taken place, but also that a portion of this metabolite is being lost to the organism. It appears from our results that free nicotinic acid is not a normal constituent in the human urine after dosing, but is found only under certain conditions, associated with the known pharmacological effects of this compound. It seems that it is due to a sudden occurrence in the organism of a high concentration of nicotinic acid which cannot be dealt with satisfactorily by the normal metabolic mechanisms. The large excretion of nicotinic acid was particularly pronounced when the dose was taken on an empty stomach. It seems probable that the liver is mainly concerned -with the formation of the glycine conjugate, and if it cannot perform this function free nicotinic acid circulating in the blood causes the vasodilatory symptoms and is ultimately excreted in the urine. The appearance of free nicotinic acid in the urine may possibly be a test for the efficiency of liver function, although the effect on the vessels of the kidney may be a complicating factor."
"Our results on human urines are in keeping with those of Holman & de Lange (1950a) who found that ingestion of nicotinic acid resulted in a greater excretion of non-methylated derivatives of nicotinic acid than after ingestion of nicotinamide."
"The spectrum of metabolites appearing in human urines after dosing with nicotinamide was different from that found after ingestion of nicotinic acid. N'-Methylnicotinamide appeared to be the major excretion product after dosing with nicotinamide, but we have no data for N1-methyl-2-pyridone-5-carbonamide which is known to be an important metabolite (Knox & Grossman, 1946, 1947; Holman & de Lange, 1949). Of the tertiary nicotinyl compounds only nicotinamide was found in the urine. It thus seems that no appreciable deamidation occurred, sufficient to cause an excretion of free nicotinic acid or its glycine conjugate."
"While only 50% of the dose of nicotinamide appeared in urines of deficient rats, almost the entire dose was recovered in the normal animals, the major part as N1-methylnicotinamide. Similarly, a higher proportion of the dose of nicotinic acid was excreted by the normal than the deficient group. It would appear that in the presence of sufficient dietary tryptophan the rat either has preference for the tryptophan-nicotinic acid conversion or the ingestion of nicotinyl compounds may influence favourably this conversion of tryptophan. This may be the reason why in normal rats such a high recovery of the dose of nicotinic acid or amide was observed. This is in agreement with the findings of Spector (1948)."
"Our results on human urines are in keeping with those of Holman & de Lange (1950a) who found that ingestion of nicotinic acid resulted in a greater excretion of non-methylated derivatives of nicotinic acid than after ingestion of nicotinamide."
"The spectrum of metabolites appearing in human urines after dosing with nicotinamide was different from that found after ingestion of nicotinic acid. N'-Methylnicotinamide appeared to be the major excretion product after dosing with nicotinamide, but we have no data for N1-methyl-2-pyridone-5-carbonamide which is known to be an important metabolite (Knox & Grossman, 1946, 1947; Holman & de Lange, 1949). Of the tertiary nicotinyl compounds only nicotinamide was found in the urine. It thus seems that no appreciable deamidation occurred, sufficient to cause an excretion of free nicotinic acid or its glycine conjugate."
"While only 50% of the dose of nicotinamide appeared in urines of deficient rats, almost the entire dose was recovered in the normal animals, the major part as N1-methylnicotinamide. Similarly, a higher proportion of the dose of nicotinic acid was excreted by the normal than the deficient group. It would appear that in the presence of sufficient dietary tryptophan the rat either has preference for the tryptophan-nicotinic acid conversion or the ingestion of nicotinyl compounds may influence favourably this conversion of tryptophan. This may be the reason why in normal rats such a high recovery of the dose of nicotinic acid or amide was observed. This is in agreement with the findings of Spector (1948)."
- Nicotinamide supplementation induces detrimental metabolic and epigenetic changes in developing rats
"Excessive intake of nicotinamide is very common in the general population primarily due to widespread niacin fortification and the use of nicotinamide supplements in foods."
"In humans, excess nicotinamide has long been known to be degraded mainly through S-adenosylmethionine-dependent methylation catalysed by nicotinamide N-methyltransferase (14). Obviously, excess nicotinamide intake will increase the consumption of limited labile methyl-group resources and may thus affect other S-adenosylmethionine-dependent methylation reactions, presumably including DNA methylation. To date, little is known about the effect of long-term excess nicotinamide intake on DNA methylation, although nicotinamide has been used to fortify foods for many decades."
"[..]low doses of nicotinamide supplementation could promote weight gain but that high doses could not. These observations are similar to those of previous studies (20, 21). The effects of excess nicotinamide exposure on rat weight gain may involve insulin resistance and oxidative stress. Our previous study found that nicotinamide degradation-induced generation of reactive oxygen species and subsequent insulin resistance could enhance the effect of glucose on insulin secretion and cause reactive hypoglycaemia (5), a major appetite-stimulating factor (22). This suggests that nicotinamide-induced weight gain may involve an increase in appetite. Indeed, low doses of nicotinamide supplementation have been demonstrated to increase food intake in rats (20, 21)."
"Methylation is a key mechanism for the degradation of nicotinamide and xenobiotics (13). The present finding that nicotinamide supplementation was associated with a dose-dependent decrease in plasma betaine levels but an increase in choline levels suggests that the methyl groups used for nicotinamide methylation are derived mainly from betaine rather than from choline. The decreased utilisation of choline may be due to the oxidative injury of the liver and kidneys, which are responsible for the conversion of choline to betaine (28). Thus, it appears that under oxidative stress conditions, betaine is an effective methyl donor, but choline is not. This may help explain why the metabolic syndrome, which is characterised by oxidative stress (29, 30), is associated with high plasma choline levels and low betaine levels (31)."
"Although nicotinamide supplementation induced a significant increase in S-adenosylmethionine-mediated methyl transfer reactions (which is indicated by a significant rise in plasma N1-methylnicotinamide levels and a decrease in plasma betaine levels), there was no significant increase in fasting plasma homocysteine levels or even a slight decrease in those of the low-dose-treated group (Fig. 3(e)). These findings suggest that there is an increase in homocysteine turnover rate in response to increased nicotinamide methylation and indicate that plasma homocysteine levels are a less sensitive indicator of disturbed methyl metabolism than plasma betaine levels. The finding that nicotinamide supplementation induced a decrease in the expression of Bhmt and Cbs but a slight increase in that of Mtr suggests that the increased homocysteine turnover may be mediated by Mtr-mediated conversion of homocysteine to methionine, in which increased catabolism of betaine may play a role because this pathway uses the metabolites of betaine as one-carbon donors (Fig. 1)."
"As has been found in the present study, excess nicotinamide exposure can induce the expression of certain genes. For example, rats fed with the nicotinamide-supplemented diets exhibited high plasma nicotinamide levels, which were associated with decreased methylation in the Nnmt promoter region and increased Nnmt expression, while the decreased plasma homocysteine levels in the low-dose nicotinamide (1 g/kg diet)-treated rats were associated with increased methylation in the Cbs promoter region and decreased Cbs expression."
"It is worth noting that excess nicotinamide-induced methyl-group depletion can induce DNA hypomethylation. The present findings, together with our previous observations that excess nicotinamide exposure could induce oxidative stress and insulin resistance (5, 33) and disturb the methylation-mediated degradation/inactivation of catecholamines (13), suggest that high nicotinamide intake may play a role in the development of oxidative stress-, insulin resistance- and methylation-related disorders."
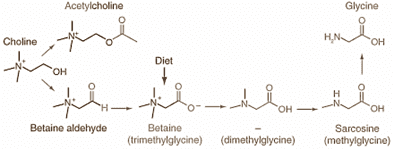
"Another interesting finding of the present study is that nicotinamide supplementation significantly reduces uracil content in rat hepatic DNA. A possible explanation for this may involve deoxythymidine monophosphate synthesis. As shown in Fig. 1, dimethylglycine, sarcosine and glycine, which may be derived from betaine catabolism, are involved in the formation of 5,10-methylenetetrahydrofolate, an essential cofactor for the synthesis of deoxythymidine monophosphate from deoxyuridine monophosphate. Thus, the increased catabolism of betaine induced by nicotinamide supplementation could increase the levels of its metabolites, which may facilitate the synthesis of 5,10-methylenetetrahydrofolate-dependent deoxythymidine monophosphate and consequently reduce uracil incorporation into DNA during DNA replication."
"In humans, excess nicotinamide has long been known to be degraded mainly through S-adenosylmethionine-dependent methylation catalysed by nicotinamide N-methyltransferase (14). Obviously, excess nicotinamide intake will increase the consumption of limited labile methyl-group resources and may thus affect other S-adenosylmethionine-dependent methylation reactions, presumably including DNA methylation. To date, little is known about the effect of long-term excess nicotinamide intake on DNA methylation, although nicotinamide has been used to fortify foods for many decades."
"[..]low doses of nicotinamide supplementation could promote weight gain but that high doses could not. These observations are similar to those of previous studies (20, 21). The effects of excess nicotinamide exposure on rat weight gain may involve insulin resistance and oxidative stress. Our previous study found that nicotinamide degradation-induced generation of reactive oxygen species and subsequent insulin resistance could enhance the effect of glucose on insulin secretion and cause reactive hypoglycaemia (5), a major appetite-stimulating factor (22). This suggests that nicotinamide-induced weight gain may involve an increase in appetite. Indeed, low doses of nicotinamide supplementation have been demonstrated to increase food intake in rats (20, 21)."
"Methylation is a key mechanism for the degradation of nicotinamide and xenobiotics (13). The present finding that nicotinamide supplementation was associated with a dose-dependent decrease in plasma betaine levels but an increase in choline levels suggests that the methyl groups used for nicotinamide methylation are derived mainly from betaine rather than from choline. The decreased utilisation of choline may be due to the oxidative injury of the liver and kidneys, which are responsible for the conversion of choline to betaine (28). Thus, it appears that under oxidative stress conditions, betaine is an effective methyl donor, but choline is not. This may help explain why the metabolic syndrome, which is characterised by oxidative stress (29, 30), is associated with high plasma choline levels and low betaine levels (31)."
"Although nicotinamide supplementation induced a significant increase in S-adenosylmethionine-mediated methyl transfer reactions (which is indicated by a significant rise in plasma N1-methylnicotinamide levels and a decrease in plasma betaine levels), there was no significant increase in fasting plasma homocysteine levels or even a slight decrease in those of the low-dose-treated group (Fig. 3(e)). These findings suggest that there is an increase in homocysteine turnover rate in response to increased nicotinamide methylation and indicate that plasma homocysteine levels are a less sensitive indicator of disturbed methyl metabolism than plasma betaine levels. The finding that nicotinamide supplementation induced a decrease in the expression of Bhmt and Cbs but a slight increase in that of Mtr suggests that the increased homocysteine turnover may be mediated by Mtr-mediated conversion of homocysteine to methionine, in which increased catabolism of betaine may play a role because this pathway uses the metabolites of betaine as one-carbon donors (Fig. 1)."
"As has been found in the present study, excess nicotinamide exposure can induce the expression of certain genes. For example, rats fed with the nicotinamide-supplemented diets exhibited high plasma nicotinamide levels, which were associated with decreased methylation in the Nnmt promoter region and increased Nnmt expression, while the decreased plasma homocysteine levels in the low-dose nicotinamide (1 g/kg diet)-treated rats were associated with increased methylation in the Cbs promoter region and decreased Cbs expression."
"It is worth noting that excess nicotinamide-induced methyl-group depletion can induce DNA hypomethylation. The present findings, together with our previous observations that excess nicotinamide exposure could induce oxidative stress and insulin resistance (5, 33) and disturb the methylation-mediated degradation/inactivation of catecholamines (13), suggest that high nicotinamide intake may play a role in the development of oxidative stress-, insulin resistance- and methylation-related disorders."
"Another interesting finding of the present study is that nicotinamide supplementation significantly reduces uracil content in rat hepatic DNA. A possible explanation for this may involve deoxythymidine monophosphate synthesis. As shown in Fig. 1, dimethylglycine, sarcosine and glycine, which may be derived from betaine catabolism, are involved in the formation of 5,10-methylenetetrahydrofolate, an essential cofactor for the synthesis of deoxythymidine monophosphate from deoxyuridine monophosphate. Thus, the increased catabolism of betaine induced by nicotinamide supplementation could increase the levels of its metabolites, which may facilitate the synthesis of 5,10-methylenetetrahydrofolate-dependent deoxythymidine monophosphate and consequently reduce uracil incorporation into DNA during DNA replication."
- Nicotinamide N-Methyltransferase: More Than a Vitamin B3 Clearance Enzyme
"NNMT (EC2.1.1.1) catalyzes the methylation of NAM and structurally related compounds using the universal methyl donor S-adenosyl methionine (Met) (SAM) to produce S-adenosyl-L-homocysteine (SAH) and N1-methylnicotinamide (MNAM) [1]. The product of NNMT, MNAM, can be further oxidized by aldehyd oxidase (Aox) into two related compounds, N1-methyl-2-pyridone-5-carboxamide (2py) and N1-methyl-4-pyridone-3-carboxamide (4py), and all three metabolites are eventually excreted in the urine [2] (Figure 1). A secondary NAM clearance pathway starts with the direct oxidation of NAM to NAM N-oxide by cyp2E1 followed by elimination in the urine [3]. Under most conditions methylation is quantitatively by far the predominant NAM clearance pathway, with the exception of an acute pharmacological dose of NAM, which is mainly converted to NAM N-oxide [4,5]."
"Cantoni correctly deduced that ATP and Met form a high-energy intermediate used for the methylation of NAM. He went on to identify SAM as the universal methyl donor a year later [10]."
"NNMT is not saturated under normal conditions and increased dietary NAM intake leads to a proportional increase in NAM methylation [14]. Because NAM methylation is irreversible, early studies focused on methyl donor depletion by high doses of NAM."
"In adult rodents depletion of methyl donors by high doses of NAM increased liver steatosis only when diet was limiting in choline. This is because choline can donate its methyl group and prevent depletion of methyl donors, which are needed for lipid export from the liver [14–17]."
"With advances in microarray and proteomic techniques, it became apparent that NNMT expression is increased in a wide variety of cancers. NNMT mRNA is increased in primary glioblastoma tumors compared with normal brain samples [61], in human papillary thyroid cancer cell lines [62], in renal clear cell carcinoma [63], and in bladder cancer [64]. The NNMT protein is increased ingastric cancers [65,66], human colorectal cancers [67], and oral squamous cell carcinoma [68]. One notable exception is hepato cellular carcinoma, where NNMT expression is suppressed [69]. Soon after the connection between NNMT and cancer was described, a causal role for NNMT in various cancer phenotypes was demonstrated. NNMT knockdown decreased the proliferation and migration of 253J laval bladder cancer cells [64]. Similarly, NNMT knockdown inhibited the proliferation and/or metastasis of renal clear cell carcinoma cells, Panc-1 pancreatic cancer cells, oral squamous carcinoma, and others [70–72]. Collectively these data support the notion that NNMT overexpression enhances the aggressiveness of various cancers."
"NNMT is strongly expressed in the adipose tissue of humans and rodents."
"Human adipose tissue NNMT expression correlates positively with adiposity and insulin resistance [18,19]. Consistent with these correlations, antisense NNMT knockdown (NNMTASO) in vivo protected C57BL6 mice from fat accumulation on a HFD and improved their glucose tolerance compared with controls [38]. NNMT knockdown occurred in both the liver and fat, but the lean metabolic phenotype appears to be mediated by increased energy expenditure by the adipose tissue. Because NNMT is a major methyltransferase in adipocytes, the authors measured the SAM/SAH ratio. NNMT knockdown increased the SAM/SAH ratio and global histone H3K4 methylation, the latter associated with transcriptional activation [38]."
"[..]Two recent studies extend these findings to humans and show that adipose tissue NNMT and its circulating product MNAM correlate positively with insulin resistance and body mass index (BMI) [18,19]."
"NNMT is not considered a major methyltransferase activity in the liver. The majority of methyl donors in the liver, where most of the methylation in the body occurs, are used for the methylation of guanidinoacetic acid (GAA) to creatine by guanidinoacetate N-methyltransferase (GAMT) and for the methylation of phosphatidylethanolamine (PE) to phosphatidylcholine (PC) by phosphatidylethanolamine N-methyltransferase (PEMT) [77,78]. Consistent with this, NNMT knockdown did not change the SAM/SAH ratio in the liver of mice or primary hepatocytes [20]. As mentioned previously, high doses of the NNMT substrate NAM caused liver SAM depletion but only on diets with limiting methyl donors [17]."
"Along the same lines, it has recently been reported that exogenous NAM lowers liver SAM content in mice null for the enzyme glycine N-methyltransferase (GNMT) (GNMT-KO mice). GNMT is a major liver methyltransferase that methylates glycine to form sarcosine, a metabolite currently without known physiological function. GNMT activity protects the liver from changes in SAM fluxes. During periods of high SAM consumption, GNMT activity declines, restoring SAM levels, and vice and versa [78]. This homeostatic mechanism is lacking in the liver of the GNMT-KO mice, which accumulate massive amounts of SAM that eventually cause DNA and proteid hypermethylation and metabolic disturbances [79]. Exogenous NAM used by NNMT lowers the liver SAM content of GNMT-KO mice, thus improving their phenotype, but does not change SAM levels in control mice [80]. Collectively, these results suggest that NNMT activity is not sufficient to alter liver methyl donor balance under normal conditions but can do so in instances of substrate overdose and compromised methyl donor recycling (Box 3)."
"It is currently assumed that consumption of methyl donors by NNMT lowers the SAM/SAH ratio and drives the changes in histone methylation. Although this is plausible, estimates suggest that less than 1% of the total urinary loss of methyl groups can be attributed to NNMT activity (75 mmol/day for MNAM+2py+4py versus 15 mmol total methyl group loss/day) [78,84], so the findings that NNMT can alter global methyl donor balance in several cell types could not have been anticipated based on its contribution to whole-body methyl group loss and requires further experimentation. This is an important point to clarify, since epigenetic marks can be modified independently of changes in methyl donors."
"Cantoni correctly deduced that ATP and Met form a high-energy intermediate used for the methylation of NAM. He went on to identify SAM as the universal methyl donor a year later [10]."
"NNMT is not saturated under normal conditions and increased dietary NAM intake leads to a proportional increase in NAM methylation [14]. Because NAM methylation is irreversible, early studies focused on methyl donor depletion by high doses of NAM."
"In adult rodents depletion of methyl donors by high doses of NAM increased liver steatosis only when diet was limiting in choline. This is because choline can donate its methyl group and prevent depletion of methyl donors, which are needed for lipid export from the liver [14–17]."
"With advances in microarray and proteomic techniques, it became apparent that NNMT expression is increased in a wide variety of cancers. NNMT mRNA is increased in primary glioblastoma tumors compared with normal brain samples [61], in human papillary thyroid cancer cell lines [62], in renal clear cell carcinoma [63], and in bladder cancer [64]. The NNMT protein is increased ingastric cancers [65,66], human colorectal cancers [67], and oral squamous cell carcinoma [68]. One notable exception is hepato cellular carcinoma, where NNMT expression is suppressed [69]. Soon after the connection between NNMT and cancer was described, a causal role for NNMT in various cancer phenotypes was demonstrated. NNMT knockdown decreased the proliferation and migration of 253J laval bladder cancer cells [64]. Similarly, NNMT knockdown inhibited the proliferation and/or metastasis of renal clear cell carcinoma cells, Panc-1 pancreatic cancer cells, oral squamous carcinoma, and others [70–72]. Collectively these data support the notion that NNMT overexpression enhances the aggressiveness of various cancers."
"NNMT is strongly expressed in the adipose tissue of humans and rodents."
"Human adipose tissue NNMT expression correlates positively with adiposity and insulin resistance [18,19]. Consistent with these correlations, antisense NNMT knockdown (NNMTASO) in vivo protected C57BL6 mice from fat accumulation on a HFD and improved their glucose tolerance compared with controls [38]. NNMT knockdown occurred in both the liver and fat, but the lean metabolic phenotype appears to be mediated by increased energy expenditure by the adipose tissue. Because NNMT is a major methyltransferase in adipocytes, the authors measured the SAM/SAH ratio. NNMT knockdown increased the SAM/SAH ratio and global histone H3K4 methylation, the latter associated with transcriptional activation [38]."
"[..]Two recent studies extend these findings to humans and show that adipose tissue NNMT and its circulating product MNAM correlate positively with insulin resistance and body mass index (BMI) [18,19]."
"NNMT is not considered a major methyltransferase activity in the liver. The majority of methyl donors in the liver, where most of the methylation in the body occurs, are used for the methylation of guanidinoacetic acid (GAA) to creatine by guanidinoacetate N-methyltransferase (GAMT) and for the methylation of phosphatidylethanolamine (PE) to phosphatidylcholine (PC) by phosphatidylethanolamine N-methyltransferase (PEMT) [77,78]. Consistent with this, NNMT knockdown did not change the SAM/SAH ratio in the liver of mice or primary hepatocytes [20]. As mentioned previously, high doses of the NNMT substrate NAM caused liver SAM depletion but only on diets with limiting methyl donors [17]."
"Along the same lines, it has recently been reported that exogenous NAM lowers liver SAM content in mice null for the enzyme glycine N-methyltransferase (GNMT) (GNMT-KO mice). GNMT is a major liver methyltransferase that methylates glycine to form sarcosine, a metabolite currently without known physiological function. GNMT activity protects the liver from changes in SAM fluxes. During periods of high SAM consumption, GNMT activity declines, restoring SAM levels, and vice and versa [78]. This homeostatic mechanism is lacking in the liver of the GNMT-KO mice, which accumulate massive amounts of SAM that eventually cause DNA and proteid hypermethylation and metabolic disturbances [79]. Exogenous NAM used by NNMT lowers the liver SAM content of GNMT-KO mice, thus improving their phenotype, but does not change SAM levels in control mice [80]. Collectively, these results suggest that NNMT activity is not sufficient to alter liver methyl donor balance under normal conditions but can do so in instances of substrate overdose and compromised methyl donor recycling (Box 3)."
"It is currently assumed that consumption of methyl donors by NNMT lowers the SAM/SAH ratio and drives the changes in histone methylation. Although this is plausible, estimates suggest that less than 1% of the total urinary loss of methyl groups can be attributed to NNMT activity (75 mmol/day for MNAM+2py+4py versus 15 mmol total methyl group loss/day) [78,84], so the findings that NNMT can alter global methyl donor balance in several cell types could not have been anticipated based on its contribution to whole-body methyl group loss and requires further experimentation. This is an important point to clarify, since epigenetic marks can be modified independently of changes in methyl donors."
- The Metabolism of Radioactive Nicotinic Acid and Nicotinamide
- https://raypeatforum.com/community/threads/incomprehensive-ble-notes-on-choline.23228/#post-329705
Last edited:
Amazoniac
Member
Forgot this:
"Nicotinamide and nicotinic acid obtained at low doses are readily absorbed and retained by the body, whereas at high doses, they are transiently absorbed and rapidly eliminated from the body, albeit with transient increases in NAD+ levels in tissues such as the liver. Two-week treatment of rats with high doses of nicotinic acid and nicotinamide (500 and 1000 mg kg–1) has been evaluated on NAD+ levels in various tissues (Jackson et al., 1995). Both blood (packed red blood cells) and liver were responsive to increased dosages of nicotinamide or nicotinic acid, leading to increases of 40 to 60% in NAD+ content for both tissues for either B3. Smaller increases in NAD+ concentrations not exceeding 15% were observed for 1000 mg kg–1 doses of nicotinamide in heart, lung, and kidneys. These findings, on the one hand, appear to confirm that nampt/PBEF activity, which is responsible for recycling nicotinamide to NAD+, is typically not rate-limited by nicotinamide concentrations in some but not all tissues."
"In cell culture, nampt/PBEF controls NAD+ concentrations independent of exogenous nicotinamide concentrations (Revollo et al., 2004). nampt/PBEF has a very low Km for nicotinamide (<2 μM), suggesting that it is readily saturated by endogenous nicotinamide concentrations (Revollo et al., 2004). On the other hand, the ability of nicotinamide to stimulate NAD+ synthesis in liver and blood suggests that nicotinamide is convertible to alternative forms of B3 that ultimately increase nicotinamide bioavailability and/or that nicotinamide treatment causes cellular adaptations that lead to improved NAD+ biosynthesis. Why nicotinamide is efficiently utilized in some but not all tissues for NAD+ biosynthesis is currently unexplained."
"Jackson et al. (1995) also showed that nicotinic acid increases NAD+ concentrations in liver and blood, similar to nicotinamide. In addition, NAD+ biosynthesis was increased in heart (50%) and kidney (100%) as well. These results show that nicotinic acid generally has a broader effect than nicotinamide for NAD+ increases in the body. These results also indicate that the Preiss-Handler pathway is typically operating below saturation in most tissues."
"Nicotinamide and nicotinic acid obtained at low doses are readily absorbed and retained by the body, whereas at high doses, they are transiently absorbed and rapidly eliminated from the body, albeit with transient increases in NAD+ levels in tissues such as the liver. Two-week treatment of rats with high doses of nicotinic acid and nicotinamide (500 and 1000 mg kg–1) has been evaluated on NAD+ levels in various tissues (Jackson et al., 1995). Both blood (packed red blood cells) and liver were responsive to increased dosages of nicotinamide or nicotinic acid, leading to increases of 40 to 60% in NAD+ content for both tissues for either B3. Smaller increases in NAD+ concentrations not exceeding 15% were observed for 1000 mg kg–1 doses of nicotinamide in heart, lung, and kidneys. These findings, on the one hand, appear to confirm that nampt/PBEF activity, which is responsible for recycling nicotinamide to NAD+, is typically not rate-limited by nicotinamide concentrations in some but not all tissues."
"In cell culture, nampt/PBEF controls NAD+ concentrations independent of exogenous nicotinamide concentrations (Revollo et al., 2004). nampt/PBEF has a very low Km for nicotinamide (<2 μM), suggesting that it is readily saturated by endogenous nicotinamide concentrations (Revollo et al., 2004). On the other hand, the ability of nicotinamide to stimulate NAD+ synthesis in liver and blood suggests that nicotinamide is convertible to alternative forms of B3 that ultimately increase nicotinamide bioavailability and/or that nicotinamide treatment causes cellular adaptations that lead to improved NAD+ biosynthesis. Why nicotinamide is efficiently utilized in some but not all tissues for NAD+ biosynthesis is currently unexplained."
"Jackson et al. (1995) also showed that nicotinic acid increases NAD+ concentrations in liver and blood, similar to nicotinamide. In addition, NAD+ biosynthesis was increased in heart (50%) and kidney (100%) as well. These results show that nicotinic acid generally has a broader effect than nicotinamide for NAD+ increases in the body. These results also indicate that the Preiss-Handler pathway is typically operating below saturation in most tissues."
Amazoniac
Member
A very interesting experiment in humans:
(for detailed results, graphs and diagrams, read it directly)
- Comparison of the effects of nicotinic acid and nicotinamide degradation on plasma betaine and choline levels - ScienceDirect
- Nicotinamide supplementation induces detrimental metabolic and epigenetic changes in developing rats
Ps.: there were various references to Travisord, which confirms that I'm a big fan.
(for detailed results, graphs and diagrams, read it directly)
- Comparison of the effects of nicotinic acid and nicotinamide degradation on plasma betaine and choline levels - ScienceDirect
"Nicotinic acid and nicotinamide are derived from different sources: the former is from plant-based foods, and the latter is from animal-based foods. Moreover, there are differences between the degradation of nicotinic acid and nicotinamide. N-methylation, catalysed by nicotinamide N-methyltransferase (NNMT), is the key enzyme first degrades nicotinamide to N1-methylnicotinamide (NMN). NMN is further oxidized to N1-methyl-2-pyridone-5-carboxamide (2-Py) and N1-methyl-4-pyridone-5-carboxamide (4-Py) by aldehyde oxidase 1 (Fig. 1). On the other hand, a considerable amount of nicotinic acid is converted to nicotinuric acid. Since methylation is a methyl-group-consuming reaction, accompanied by the production of homocysteine (Hcy), it is assumed that nicotinic acid and nicotinamide at the same doses may have different effects on the pool size of labile methyl groups, which is also a prerequisite for the methylation of numerous other substrates, including DNA and catecholamines (epinephrine and norepinephrine)."
"Niacin fortification is associated with increased prevalence of diabetes with a lag time of 26 years [7], while high-dose nicotinic acid therapy is found to be associated with a rapid increase in diabetes rates (within years) [3,4]."
"It has been reported in animal studies that excess nicotinamide causes DNA hypomethylation, an important epigenetic alteration in diseases [12,13]. It is therefore of significance to investigate the effects of high niacin intake on the methyl pool in the human body."
..
"The major findings of the present study are that nicotinamide has more profound effects on the levels of plasma betaine, NMN, Hcy, methylated metabolites of catecholamines, urinary 2-Py excretion and PP than nicotinic acid at the same dose."
"According to the functions of components in the methylation process, they can be divided into four categories: substrates, methyl-group donors, methylation mediators and enzymes involved in one-carbon unit transfer reactions. Substrates, derived from both exogenous and endogenous sources (e.g. niacin, catecholamines and DNA), are methyl consumers. Methyl-group (one-carbon unit) donors are compounds that provide methyl groups for the methylation of substrates, and they include choline, betaine, dimethylglycine, sarcosine and glycine, which are non-renewable resources. Methylation mediators, which can be recycled in methylation reactions, are compounds that mediate the transfer of methyl groups/one-carbon units from methyl donors to methyl consumers. Evidently, an increase in substrate levels will increase the demand for methyl donors rather than methylation mediators. This may explain the association between the dose of nicotinamide and the level of plasma betaine [13], and the association between decreased plasma betaine levels and increased NMN levels in the NM and NA groups. The methylation of nicotinamide and nicotinic acid increases the consumption of methyl groups. This effect can disturb the methylation of other substrates, such as the methylation of catecholamines and DNA, as found in our present and previous studies [12,13[above],17[Travisord's take]]. DNA hypomethylation may occur in the skin as well after long-term nicotinamide treatment, which might be somehow responsible for reducing the rates of new non-melanoma skin cancers and actinic keratoses in high-risk patients [6]."
"Methylation is accompanied by the production of Hcy, which is remethylated to methionine through either a betaine- or folate-dependent pathway when there is adequate supply of methyl donors. Thus, increased levels of plasma Hcy may reflect an increased consumption of methyl donors. Indeed, the present study found that plasma NMN and Hcy levels are both reversely associated with plasma betaine levels. Therefore, supplementation of methylation mediators (e.g. folic acid and B12) cannot prevent or reduce excess accumulation of substrate(s). This may explain why supplementation of methylation mediators (folic acid and B12) neither improve DNA methylation [18,19], nor reduce cardiovascular risk [20-22] (see Fig. 7)."
"Choline is a well-known methyl donor, but it cannot play a methyl-donor role until it is converted to betaine in the liver and kidneys [23]. This study shows that nicotinamide and nicotinic acid do not change the levels of plasma choline. In our previous study, we also found that there was a significant decrease in plasma betaine level, but no significant change in plasma choline level at 5 h after a single oral dose of nicotinamide (100 mg) [24]. Given that the degradation of nicotinamide is accompanied by the generation of reactive oxidative species (ROS) [14,15], which may reduce cellular function, it is likely that niacin may disturb choline utilisation. If this is the case, long-term high niacin intake should cause an accumulation of choline in the blood. Indeed, our previous animal studies have demonstrated that rats given high nicotinamide supplementation for 8 weeks causes oxidative injury in liver and kidneys, which was associated with decreased plasma betaine levels but increased choline levels [13]. Therefore, nicotinamide loading-induced oxidative stress causes the tissue dysfunction, which further disturbs choline utilisation. Interestingly, the association between decreased plasma betaine and increased choline levels is also observed in human metabolic syndrome [25]. Because metabolic syndrome is associated with oxidative stress (or elevated ROS levels) [26], it is likely that oxidative stress can decrease the utilisation of choline as a methyl donor."
"Betaine is closely related to the metabolism of other methyl donors, choline (its precursor), dimethylglycine, sarcosine and glycine (which are its metabolites), as shown in Fig. 6. As an intracellular osmolyte, betaine presents in high concentrations in the liver and kidneys, two major detoxifying organs in the body [27]. Most importantly, unlike other one-carbon donors, betaine donates a methyl group directly to Hcy to form methionine. The present findings that there is an inverse relationship between plasma betaine and NMN levels in the NM and NA groups suggest that betaine plays an important role in the degradation of niacin."
"The metabolites of betaine may contribute to the formation of 5-methyltetrahydrofolate, a cosubstrate for Hcy remethylation to methionine. According to the relationship shown in Fig. 6, the complete catabolism of one molecule of betaine can help regenerate four molecules of methionine from Hcy and thus, in theory, can methylate four molecules of substrate. However, our previous study [12] found that a maternal supplementation of 2.0 g betaine per kilogram diet only partially prevents nicotinamide-induced (4.0 g/kg diet) decrease in DNA methylation in foetal [sick] rat liver, despite their similar formula weight (FW(betaine) = 117.15; FW(nicotinamide) = 122.12). Thus, it appears that betaine may contribute mainly to the methylation of dietary substrates via betaine-dependent Hcy remethylation, as proposed by Olthof and Verhoef [28], and its metabolites may contribute mainly to deoxythymidine monophosphate (dTMP) synthesis, presumably by affecting the synthesis of 5,10-methylenetetrahydrofolate. Indeed, in our previous studies, we found that besides reducing DNA methylation, nicotinamide supplementation also reduces uracil levels in DNA, which involves an increase in the synthesis of dTMP from deoxyuridine monophosphate (dUMP) [another one!] [12]. This may explain why betaine reduces the increase in Hcy after methionine loading, whereas folic acid has no effect [29]."
Aha!"Niacin fortification is associated with increased prevalence of diabetes with a lag time of 26 years [7], while high-dose nicotinic acid therapy is found to be associated with a rapid increase in diabetes rates (within years) [3,4]."
"It has been reported in animal studies that excess nicotinamide causes DNA hypomethylation, an important epigenetic alteration in diseases [12,13]. It is therefore of significance to investigate the effects of high niacin intake on the methyl pool in the human body."
..
"The major findings of the present study are that nicotinamide has more profound effects on the levels of plasma betaine, NMN, Hcy, methylated metabolites of catecholamines, urinary 2-Py excretion and PP than nicotinic acid at the same dose."
"According to the functions of components in the methylation process, they can be divided into four categories: substrates, methyl-group donors, methylation mediators and enzymes involved in one-carbon unit transfer reactions. Substrates, derived from both exogenous and endogenous sources (e.g. niacin, catecholamines and DNA), are methyl consumers. Methyl-group (one-carbon unit) donors are compounds that provide methyl groups for the methylation of substrates, and they include choline, betaine, dimethylglycine, sarcosine and glycine, which are non-renewable resources. Methylation mediators, which can be recycled in methylation reactions, are compounds that mediate the transfer of methyl groups/one-carbon units from methyl donors to methyl consumers. Evidently, an increase in substrate levels will increase the demand for methyl donors rather than methylation mediators. This may explain the association between the dose of nicotinamide and the level of plasma betaine [13], and the association between decreased plasma betaine levels and increased NMN levels in the NM and NA groups. The methylation of nicotinamide and nicotinic acid increases the consumption of methyl groups. This effect can disturb the methylation of other substrates, such as the methylation of catecholamines and DNA, as found in our present and previous studies [12,13[above],17[Travisord's take]]. DNA hypomethylation may occur in the skin as well after long-term nicotinamide treatment, which might be somehow responsible for reducing the rates of new non-melanoma skin cancers and actinic keratoses in high-risk patients [6]."
"Methylation is accompanied by the production of Hcy, which is remethylated to methionine through either a betaine- or folate-dependent pathway when there is adequate supply of methyl donors. Thus, increased levels of plasma Hcy may reflect an increased consumption of methyl donors. Indeed, the present study found that plasma NMN and Hcy levels are both reversely associated with plasma betaine levels. Therefore, supplementation of methylation mediators (e.g. folic acid and B12) cannot prevent or reduce excess accumulation of substrate(s). This may explain why supplementation of methylation mediators (folic acid and B12) neither improve DNA methylation [18,19], nor reduce cardiovascular risk [20-22] (see Fig. 7)."
"Choline is a well-known methyl donor, but it cannot play a methyl-donor role until it is converted to betaine in the liver and kidneys [23]. This study shows that nicotinamide and nicotinic acid do not change the levels of plasma choline. In our previous study, we also found that there was a significant decrease in plasma betaine level, but no significant change in plasma choline level at 5 h after a single oral dose of nicotinamide (100 mg) [24]. Given that the degradation of nicotinamide is accompanied by the generation of reactive oxidative species (ROS) [14,15], which may reduce cellular function, it is likely that niacin may disturb choline utilisation. If this is the case, long-term high niacin intake should cause an accumulation of choline in the blood. Indeed, our previous animal studies have demonstrated that rats given high nicotinamide supplementation for 8 weeks causes oxidative injury in liver and kidneys, which was associated with decreased plasma betaine levels but increased choline levels [13]. Therefore, nicotinamide loading-induced oxidative stress causes the tissue dysfunction, which further disturbs choline utilisation. Interestingly, the association between decreased plasma betaine and increased choline levels is also observed in human metabolic syndrome [25]. Because metabolic syndrome is associated with oxidative stress (or elevated ROS levels) [26], it is likely that oxidative stress can decrease the utilisation of choline as a methyl donor."
"Betaine is closely related to the metabolism of other methyl donors, choline (its precursor), dimethylglycine, sarcosine and glycine (which are its metabolites), as shown in Fig. 6. As an intracellular osmolyte, betaine presents in high concentrations in the liver and kidneys, two major detoxifying organs in the body [27]. Most importantly, unlike other one-carbon donors, betaine donates a methyl group directly to Hcy to form methionine. The present findings that there is an inverse relationship between plasma betaine and NMN levels in the NM and NA groups suggest that betaine plays an important role in the degradation of niacin."
"The metabolites of betaine may contribute to the formation of 5-methyltetrahydrofolate, a cosubstrate for Hcy remethylation to methionine. According to the relationship shown in Fig. 6, the complete catabolism of one molecule of betaine can help regenerate four molecules of methionine from Hcy and thus, in theory, can methylate four molecules of substrate. However, our previous study [12] found that a maternal supplementation of 2.0 g betaine per kilogram diet only partially prevents nicotinamide-induced (4.0 g/kg diet) decrease in DNA methylation in foetal [sick] rat liver, despite their similar formula weight (FW(betaine) = 117.15; FW(nicotinamide) = 122.12). Thus, it appears that betaine may contribute mainly to the methylation of dietary substrates via betaine-dependent Hcy remethylation, as proposed by Olthof and Verhoef [28], and its metabolites may contribute mainly to deoxythymidine monophosphate (dTMP) synthesis, presumably by affecting the synthesis of 5,10-methylenetetrahydrofolate. Indeed, in our previous studies, we found that besides reducing DNA methylation, nicotinamide supplementation also reduces uracil levels in DNA, which involves an increase in the synthesis of dTMP from deoxyuridine monophosphate (dUMP) [another one!] [12]. This may explain why betaine reduces the increase in Hcy after methionine loading, whereas folic acid has no effect [29]."
Trimethylglycine, which is commonly called betaine, is 65% glycine. When people consume it, they're not only supplying extra methyl groups, but also providing a bit of glycine. Spinach is a brutal source, it has about 550 mg for every 100 g, which gives us [ . . . Brewing the calculator . . . ] 360 mg of glycine. Ok, never mind, the effects comes from the methyl groups indeed.
But here's how they vary:
"In summary, this study demonstrated that both nicotinic acid and nicotinamide degradation could reduce the plasma methylgroup pool size, which may increase the risk of diseases related to aberrant methylation and epigenetic alterations. As nicotinamide can lead to more consumption of betaine and more production of Hcy than nicotinic acid, it may have a higher risk of causing methylation/Hcy-related diseases."But here's how they vary:
- Nicotinamide supplementation induces detrimental metabolic and epigenetic changes in developing rats
Ps.: there were various references to Travisord, which confirms that I'm a big fan.
Last edited:
Amazoniac
Member
One more thing..
Shamans distinguish B-vitamins between those that are immediately involved in energy production:
- Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism - ScienceDirect
and those that do the participatings in ein-carbon transfer (methylation) reactions:
- Mitochondrial function and toxicity: Role of B vitamins on the one-carbon transfer pathways - ScienceDirect
When you obtain niacin from foods, it comes balanced, so there are no risks of depleting the others. Even coffee, which is a good source of nicotinic acid, also provides caffeine (with its three methyl groups*) and does the conferings of synergistic protection in the useless liver. When you take nicotinic acid alone, or caffeine alone, you have to compensate elsewhere. This is also why Gerson's (yes, I'm getting paid) approach would fail if he didn't include liver extract to his therapy and instead used supplemental nicotinic acid alone.
By the way, I don't wear one of those rainbow spiral shirts to be talking about this. It's just difficult to make supplementation work if it's not supported.
Shamans distinguish B-vitamins between those that are immediately involved in energy production:
- Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism - ScienceDirect
and those that do the participatings in ein-carbon transfer (methylation) reactions:
- Mitochondrial function and toxicity: Role of B vitamins on the one-carbon transfer pathways - ScienceDirect
When you obtain niacin from foods, it comes balanced, so there are no risks of depleting the others. Even coffee, which is a good source of nicotinic acid, also provides caffeine (with its three methyl groups*) and does the conferings of synergistic protection in the useless liver. When you take nicotinic acid alone, or caffeine alone, you have to compensate elsewhere. This is also why Gerson's (yes, I'm getting paid) approach would fail if he didn't include liver extract to his therapy and instead used supplemental nicotinic acid alone.
By the way, I don't wear one of those rainbow spiral shirts to be talking about this. It's just difficult to make supplementation work if it's not supported.
Last edited:
EMF Mitigation - Flush Niacin - Big 5 Minerals
Similar threads
- Replies
- 22
- Views
- 14K
- Replies
- 13
- Views
- 5K
- Replies
- 5
- Views
- 1K
- Replies
- 9
- Views
- 6K
- Replies
- 12
- Views
- 5K
- Replies
- 53
- Views
- 26K
- Replies
- 11
- Views
- 6K
- Replies
- 75
- Views
- 23K