Amazoniac
Member
Stabilization-oriented preformulation study of photolabile menatetrenone (vitamin K2) - ScienceDirect
"Menatetrenone (vitamin K,; 2-methyl-3-alltrans-tetraprenyl-1,Cnaphthoquinone) was originally developed in Japan and has been used as injections for the treatment of hypoprothrombinemia due to a lack of vitamin K in newborns. Because the drug is very photolabile, it is necessary to protect the commercial dosage form from light."
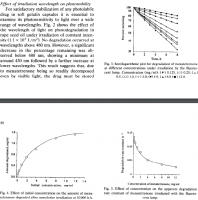
"To study the effect of specific wavelength of light on the photostability of menatetrenone, the same grating monochromator as described in our previous paper (Matsuda et al., 1989) was used within the wavelength range of 290-537 nm. Samples were exposed to light at each wavelength until the intensity level of the irradiation reached the same value (1.1 X 10^5 J/m^2). In the ordinary stability tests a white fluorescent lamp (type FL20SW, Toshiba Co., Japan) was used as a light source."
"No degradation occurred at wavelengths above 480 nm. However, a significant decrease in the percentage remaining was observed below 480 nm, showing a minimum at around 430 nm followed by a further increase at lower wavelengths. This result suggests that, due to menatetrenone being so readily decomposed even by visible light, the drug must be stored carefully if a fluorescent lamp is used in a stock room."

"A slight increase in degradation rate constant was found as the temperature rose."
"If all light beams radiating from the fluorescent lamp are parallel, the degradation rate constant should be constant, irrespective of the exposure area. However, a significant effect of exposure area was observed, in contrast to expectations. This phenomenon might be due to scattered light straying into the sample solution, the contribution of which may be significant. In cases where the intensity of incident light was kept constant, the reason why the drug was apparently stabilized with increasing depth of solution was attributable to a lowering of the relative absorption efficiency of light energy per molecule with increasing number of drug molecules, in addition to the supply of light energy to the lower parts of the solution being insufficient."
"[..]the photostabilizing design of encapsulated menatetrenone should be more satisfactorily achieved by using round capsules rather than oval, oblong, tubeshape forms, since the solid having the minimum surface area per unit volume is a sphere."
--
Electromagnetic spectrum - Wikipedia
http://onlinelibrary.wiley.com/doi/10.1111/j.1151-2916.1954.tb14006.x/epdf
Dairy - Package Protection And Preservation
Simply Red
@haidut @healthnatura
"Menatetrenone (vitamin K,; 2-methyl-3-alltrans-tetraprenyl-1,Cnaphthoquinone) was originally developed in Japan and has been used as injections for the treatment of hypoprothrombinemia due to a lack of vitamin K in newborns. Because the drug is very photolabile, it is necessary to protect the commercial dosage form from light."
"To study the effect of specific wavelength of light on the photostability of menatetrenone, the same grating monochromator as described in our previous paper (Matsuda et al., 1989) was used within the wavelength range of 290-537 nm. Samples were exposed to light at each wavelength until the intensity level of the irradiation reached the same value (1.1 X 10^5 J/m^2). In the ordinary stability tests a white fluorescent lamp (type FL20SW, Toshiba Co., Japan) was used as a light source."
"No degradation occurred at wavelengths above 480 nm. However, a significant decrease in the percentage remaining was observed below 480 nm, showing a minimum at around 430 nm followed by a further increase at lower wavelengths. This result suggests that, due to menatetrenone being so readily decomposed even by visible light, the drug must be stored carefully if a fluorescent lamp is used in a stock room."
"A slight increase in degradation rate constant was found as the temperature rose."
"If all light beams radiating from the fluorescent lamp are parallel, the degradation rate constant should be constant, irrespective of the exposure area. However, a significant effect of exposure area was observed, in contrast to expectations. This phenomenon might be due to scattered light straying into the sample solution, the contribution of which may be significant. In cases where the intensity of incident light was kept constant, the reason why the drug was apparently stabilized with increasing depth of solution was attributable to a lowering of the relative absorption efficiency of light energy per molecule with increasing number of drug molecules, in addition to the supply of light energy to the lower parts of the solution being insufficient."
"[..]the photostabilizing design of encapsulated menatetrenone should be more satisfactorily achieved by using round capsules rather than oval, oblong, tubeshape forms, since the solid having the minimum surface area per unit volume is a sphere."
--
Electromagnetic spectrum - Wikipedia
http://onlinelibrary.wiley.com/doi/10.1111/j.1151-2916.1954.tb14006.x/epdf
Dairy - Package Protection And Preservation
Simply Red
@haidut @healthnatura
Last edited: