TreasureVibe
Member
- Joined
- Jul 3, 2016
- Messages
- 1,941
Hi all. I was wondering, if Ray Peat has ever mentioned IP6. I see it's a subsection here on the Ray Peat forum. Also does anyone know if there are risks related to its use, like anemia? And I've read an anecdotal report before that stated IP6 succesfully chelated cadmium in a person down to zero on his test. Perhaps anyone knows more about IP6's chelating properties? IP6 is also touted in studies as a potent anti-cancer agent, which instead of killing the cancer cells, reverts them back to the normal phenotype. Because of it's natural function to chelate iron, this too aids in cancer because tumors require iron.
It is touted as a vitamin on its Wikipedia page:
"The study concludes that: "Given the numerous health benefits, phytates participation in important intracellular biochemical pathways, normal physiological presence in our cells, tissues, plasma, urine, etc., the levels of which fluctuate with intake, epidemiological correlates of phytate deficiency with disease and reversal of those conditions by adequate intake, and safety – all strongly suggest for phytates inclusion as an essential nutrient, perhaps a vitamin."
Source: Phytic acid - Wikipedia
Inositol is apparently made in the body as well:
"It is made naturally in human beings from glucose. Each kidney makes 2g a day; so 4g a day total is made. Other tissues synthesize it too, and the highest concentration is in the brain where it plays an important role making other neurotransmitters, and some steroid hormones bind to their receptors.[3]"
Source: Inositol - Wikipedia
However, phytic acid seems to have a possible negative side as well:
Phytic acid is one of a number of “anti-nutrients” in grains and legumes. For an introduction to this subject, please see this article. Proper preparation of whole grains will neutralize a large portion of these problematic compounds.
Studies on phytic acid reveal that for some people, the phytic acid in whole grains blocks calcium, zinc, magnesium, iron and copper; others seem immune to these adverse consequences, probably because of favorable gut flora, which in some cases can break down phytic acid. In addition, when animal fats providing vitamins A and D accompany dietary whole grains, the effects of phytic acid are mitigated.
The author of the following article found that eliminating phytic acid in his diet and the diet of his family helped reverse serious tooth decay; not everyone will need to take such drastic steps. However, proper preparation of whole grains is a good idea for everyone as it is a practice found almost universally among nonindustrialized peoples.
Source: https://www.westonaprice.org/health-topics/vegetarianism-and-plant-foods/living-with-phytic-acid/
However, this source states that IP6 is misunderstood by dietitians and the like:
"Therefore, not only that the key misconceptions about this molecule are nullified, but we have also demonstrated that IP6 is an essential nutrient whose level in plasma and urine fluctuates following deficiency or replenishment (71). In essence, IP6 has many characteristics of a vitamin, contrary to the established and, unfortunately, still existing dogma among nutritionist about its “anti-nutrient” role.
It is our hope that these apparent conflicting thoughts about the IP6 among biochemists and nutritionists finally will be reconciled."
Source: https://www.researchgate.net/public...n_Against_Cancer_by_Dietary_IP_6_and_Inositol
And another source says:
IP6 is misunderstood
Unfortunately dietitians have been taught that IP6 is an anti-nutrient, that because of its mineral chelating properties it deprives the body of essential nutrients. Dietitians often fail to distinguish growing children, who have high calcium and iron needs, from adults who begin to accumulate excessive minerals with advancing age. Females experience delayed accumulation of iron because of monthly menstruation and avoid calcium overload by donating this mineral to their offspring during pregnancy and lactation.
While nutritionists have been taught that IP6 in whole grains and seeds suppress the bioavailability of minerals, when IP6 was added to the diet of mice it did not affect their absorption of iron or calcium. [Journal Nutrition 114: 1192-98, 1984; Journal Nutrition 111: 841-47, 1981] Another study concluded that IP6 has no negative effects on mineral status and that adequate amounts in the diet are “remarkable and must be favorably considered.” [J Trace Element Med Biology15:221-8, 2001]
Source: Riceplex Global - IP6 Inositol Hexaphosphate
I found one of the studies referenced:
https://www.researchgate.net/publication/11515306_Dietary_phytate_and_mineral_bioavailability
IP6 attaches to heavy metals such as mercury, lead and cadmium, as well as loose iron, copper and calcium. [J Agriculture Food Chemistry 47: 4714-17, 999] IP6 is a selective chelator -- it does not attach to potassium, sodium or magnesium, important electrolyte minerals required for heart rhythm. IP6 does not remove calcium from bones or iron from red blood cells. Once chelated (attached), these excess minerals are excreted via the urinary tract. [Crit Rev Food Sci Nutr. 35:495-508, 1995]
Also a fascinating article: Inositol Stops Lung Cancer in Cigarette Smokers
And another: The Overlooked Cancer Cure From Japan - LewRockwell LewRockwell.com
And another with images: Cancer and IP6 - Conners Clinic
Good article: IP6 is Highly Effective Alternative Treatment for Cancer
Populations with diets high in IP6 have low incidences of cancers
Dr. Shamsuddin's studies have shown that tumor regression takes place in people using 8 grams of IP6 daily for three to four weeks. This is the amount that would be found in 12 ounces of whole kernel corn. One of his studies found that after two weeks the tumors of mice treated three times a week with IP6 were 96 percent smaller than the tumors of mice that did not receive IP6. Populations with diets high in IP6 have lower incidences of cancers of the breast, colon and prostate. Dr. Shamsuddin's laboratory experiments with IP6 have been reproduced and extended by scientists around the world, reconfirming his amazing findings. This study is from Acta Poloniae Pharmaceutica, November through December, 2008.
IP6 is more than a cancer treatment
In addition to the anti-cancer benefits of IP6, research is revealing its benefits in treating diabetes, depression, osteoporosis, heart disease, and kidney stones. It has recently been shown to help Parkinson's patients because of its ability to chelate excess iron and thereby reduce oxidative stress that results in neuronal degradation.
Alot of research on IP6 comes from Dr. Shamsuddin. Here's an interview article about him:
https://www.healthquestpodcast.com/...-6-my-interview-with-dr-abulkalam-shamsuddin/
Quote from the article:
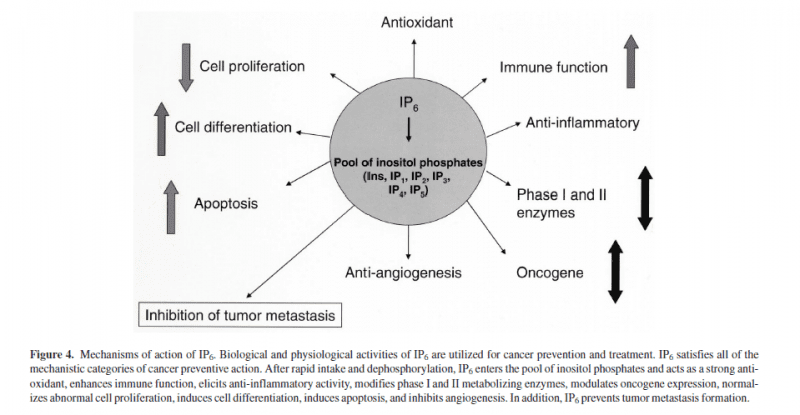
Inositol hexaphosphate (IP(6)) is a naturally occurring carbohydrate, abundantly present in many plant sources and in certain high-fiber diets, such as cereals and legumes. In addition to being found in plants, IP(6) is contained in almost all mammalian cells, although in much smaller amounts, where it is important in regulating vital cellular functions such as signal transduction, cell proliferation, and differentiation. For a long time IP(6) has been recognized as a natural antioxidant. Recently IP(6) has received much attention for its role in cancer prevention and control of experimental tumor growth, progression, and metastasis. In addition, IP(6) possesses other significant benefits for human health, such as the ability to enhance immune system, prevent pathological calcification and kidney stone formation, lower elevated serum cholesterol, and reduce pathological platelet activity. IP(6) is rapidly taken up into cells which further affect signal pathways resulting in cell cycle arrest. A striking anticancer action of IP(6) was demonstrated in different experimental models. In addition to reducing cell proliferation, IP(6) also induces differentiation of malignant cells. Enhanced immunity and antioxidant properties also contribute to tumor cell destruction. Preliminary studies in humans show that IP(6) and inositol, the precursor molecule of IP(6), appear to enhance the anticancer effect of conventional chemotherapy, control cancer metastases, and improve quality of life. Because it is abundantly present in regular diet, efficiently absorbed from the gastrointestinal tract, and safe, IP(6) + inositol holds great promise in our strategies for cancer prevention and therapy. Excerpt (Abstract: Protection against cancer by dietary IP6 and inositol. Nutr Cancer. 2006;55(2):109-25.)
About Dr. Abulkalam Shamsuddin M.B.B.S., Ph.D.
Dr Shamsuddin graduated Dhaka Medical College, University of Dhaka in 1972. Following Internship in Massachusetts and Residency training in pathology in Maryland, he was certified by the American Board of Pathology in 1977. In 1980, he received Ph.D. degree from the University of Maryland for his work on colon carcinogenesis. He joined the faculty of the University of Maryland as an Instructor in 1977, and rose through the ranks of Assistant Professor and Associate Professor to become a full Professor in 1988. For his excellence in teaching he received the Best Teacher award from the medical students many times, including the coveted “Golden Apple Award” by the American Medical Students’ Association in 1999.
Research Interests:
Dr. Shamsuddin has been studying the process of cancer formation and the prevention of cancer since 1975. His research in the steps of colon cancer formation resulted in the development of a very simple and accurate screening test for colon cancer. This rather inexpensive test detects cancers at a very early stage, and even before they have formed; in other words, it can also detect precancerous polyps and other precancerous lesions and conditions. The test has been in use in China since the early 1990’s often referred to as “Shams’ Test.” He further discovered that the colon cancer marker Gal-GalNAc is a common cancer marker expressed through a similar “field-effect” phenomenon; thus forming the basis for a general cancer screening test such as for cancer of the lungs and breast, and possibly prostate and uterine cervix.
In mid 1985, Dr. Shamsuddin started his pioneering experiments on the anti-cancer property of inositol and inositol hexaphosphate (IP-6) – natural constituents of cereals and legumes such as rice, corn, beans etc. For the next two decades, he has reconfirmed and expanded his groundbreaking studies to show the efficacy of IP-6 and inositol against different cancers in various experimental models. Dr. Shamsuddin’s book on this “IP6: Nature’s Revolutionary Cancer Fighter” by Kensington Publishing Corporation, New York, 1998, is written for the general public. Aside from these Dr. Shamsuddin has contributed numerous book-chapters and published over 200 scientific papers.
IP6 is apparently effective when combined in a good ratio with inositol, creating IP3. IP3 is very potent anti-cancer, sources state.
Here is an informative video on IP6:
And another one:
IP6 with inositol is found naturally in every body cell in the human body according to Dr. Shamsuddin. It is disputed wether or not IP6 plays a negative role in radiation therapy for cancer, as IP6 appears to stimulate the NHEJ pathway, according to the following article publishedin 2004, with its discovery of IP6 stimulating NHEJ in 2000:
Enemy Within
Dr. Shamsuddin says however, in the following article which was published in 2007 that it is protective and synergetic in radiation therapy for cancer, and that he was inspired to research this as reports from a trial in Croatia which begun in 2001 showed these protective effects during radiation therapy:
https://www.sciencedaily.com/releases/2007/11/071105083735.htm
So all in all, its role concerning radiation and radiation therapy for cancer should be further investigated, as it is disputed.
Also an informative website, by Dr. Shamsuddin himself, I think: http://www.ip-6.net/The_Science.html
So all in all, IP6 seems to be a very interesting molecule.
What do you know about IP6?
It is touted as a vitamin on its Wikipedia page:
"The study concludes that: "Given the numerous health benefits, phytates participation in important intracellular biochemical pathways, normal physiological presence in our cells, tissues, plasma, urine, etc., the levels of which fluctuate with intake, epidemiological correlates of phytate deficiency with disease and reversal of those conditions by adequate intake, and safety – all strongly suggest for phytates inclusion as an essential nutrient, perhaps a vitamin."
Source: Phytic acid - Wikipedia
Inositol is apparently made in the body as well:
"It is made naturally in human beings from glucose. Each kidney makes 2g a day; so 4g a day total is made. Other tissues synthesize it too, and the highest concentration is in the brain where it plays an important role making other neurotransmitters, and some steroid hormones bind to their receptors.[3]"
Source: Inositol - Wikipedia
However, phytic acid seems to have a possible negative side as well:
Phytic acid is one of a number of “anti-nutrients” in grains and legumes. For an introduction to this subject, please see this article. Proper preparation of whole grains will neutralize a large portion of these problematic compounds.
Studies on phytic acid reveal that for some people, the phytic acid in whole grains blocks calcium, zinc, magnesium, iron and copper; others seem immune to these adverse consequences, probably because of favorable gut flora, which in some cases can break down phytic acid. In addition, when animal fats providing vitamins A and D accompany dietary whole grains, the effects of phytic acid are mitigated.
The author of the following article found that eliminating phytic acid in his diet and the diet of his family helped reverse serious tooth decay; not everyone will need to take such drastic steps. However, proper preparation of whole grains is a good idea for everyone as it is a practice found almost universally among nonindustrialized peoples.
Source: https://www.westonaprice.org/health-topics/vegetarianism-and-plant-foods/living-with-phytic-acid/
However, this source states that IP6 is misunderstood by dietitians and the like:
"Therefore, not only that the key misconceptions about this molecule are nullified, but we have also demonstrated that IP6 is an essential nutrient whose level in plasma and urine fluctuates following deficiency or replenishment (71). In essence, IP6 has many characteristics of a vitamin, contrary to the established and, unfortunately, still existing dogma among nutritionist about its “anti-nutrient” role.
It is our hope that these apparent conflicting thoughts about the IP6 among biochemists and nutritionists finally will be reconciled."
Source: https://www.researchgate.net/public...n_Against_Cancer_by_Dietary_IP_6_and_Inositol
And another source says:
IP6 is misunderstood
Unfortunately dietitians have been taught that IP6 is an anti-nutrient, that because of its mineral chelating properties it deprives the body of essential nutrients. Dietitians often fail to distinguish growing children, who have high calcium and iron needs, from adults who begin to accumulate excessive minerals with advancing age. Females experience delayed accumulation of iron because of monthly menstruation and avoid calcium overload by donating this mineral to their offspring during pregnancy and lactation.
While nutritionists have been taught that IP6 in whole grains and seeds suppress the bioavailability of minerals, when IP6 was added to the diet of mice it did not affect their absorption of iron or calcium. [Journal Nutrition 114: 1192-98, 1984; Journal Nutrition 111: 841-47, 1981] Another study concluded that IP6 has no negative effects on mineral status and that adequate amounts in the diet are “remarkable and must be favorably considered.” [J Trace Element Med Biology15:221-8, 2001]
Source: Riceplex Global - IP6 Inositol Hexaphosphate
I found one of the studies referenced:
https://www.researchgate.net/publication/11515306_Dietary_phytate_and_mineral_bioavailability
IP6 attaches to heavy metals such as mercury, lead and cadmium, as well as loose iron, copper and calcium. [J Agriculture Food Chemistry 47: 4714-17, 999] IP6 is a selective chelator -- it does not attach to potassium, sodium or magnesium, important electrolyte minerals required for heart rhythm. IP6 does not remove calcium from bones or iron from red blood cells. Once chelated (attached), these excess minerals are excreted via the urinary tract. [Crit Rev Food Sci Nutr. 35:495-508, 1995]
Also a fascinating article: Inositol Stops Lung Cancer in Cigarette Smokers
Two famous researchers discovered inositol prevents cancer
Dr. Lee Wattenberg, known as the father of chemoprevention, searched for several decades starting in the 1970`s to find naturally occurring compounds that could theoretically prevent cancer and applied scientific methodologies to research his discoveries. After testing several molecules, he found inositol showed great promise. Using various study models he was able to demonstrate that inositol could prevent lung cancer. It had previously been documented that a poor diet increased the chances for cancer to occur, but Dr. Wattenberg was among the first to show that a common nutrient could actually prevent cancer.
A few years later, Dr. Abdul Kalam Shamsuddin, known as the father of IP6 (inositol hexaphosphate), also showed that inositol was able to prevent cancer, demonstrating the preventive value of the compound with colon cancer. Research by Dr. Shamsuddin revealed that inositol affects health in several ways, largely because it is in all human cells and is a major component of cell linings or membranes where it facilitates communication between the various organelles and molecules in the cell signaling process.
And another: The Overlooked Cancer Cure From Japan - LewRockwell LewRockwell.com
About 70% of the IP6 made by Tsuno Foods and Rice Company of Wakayama, Japan, is available to chelate (attach) to iron (as well as heavy metals), which are primary growth factors for tumors. IP6 as an extract from rice bran is a far more effective anti-cancer agent than rice bran or bran cereal alone. [Vucenik I, Nutrition Cancer 28: 7—13, 1997]
The safety record of IP6 is long standing. First, it is a normal dietary component and is found in every living cell of the body. Second, extensive studies have been conducted to confirm the lack of toxicity of IP6. In 1987 phytic acid researcher Ernst Graf reported that only 4 of 22 chelating agents studied, including IP6, block hydroxyl radical production. Only phytic acid IP6 was found to be economical, nontoxic, and effective. [Graf E, Journal Biological Chemistry 262: 11647—50, 1987]
Does it work? Case reports
Since writing a book about iron and IP6 (The Iron Time Bomb), numerous reports of dramatic cancer remissions involving this dietary supplement have been received. Some of them notably stand out.
An 80-year old man with terminal liver cancer took IP6 for a few weeks prior to a scheduled rescue procedure where an anti-tumor drug was to be injected directly into the liver. A cat scan performed just prior to the procedure revealed the liver tumor was completely necrotic — the tumor was a ball of dead cells.
A middle-aged woman whose husband worked for a prominent member of Congress, who had stage 4 breast cancer, experienced a rapid and complete remission following the consumption of IP6.
At age 70, a man was diagnosed with lung cancer. Radiologists had missed a lung tumor the size of a golf ball in an earlier x-ray. A year later it was the size of a softball. Chemotherapy reduced the tumor by 75 percent. In 1999 the man began taking IP6. By 2004 the lung tumor had completely disappeared, which was confirmed by bronchoscopy and x-ray.
A man with recurrent bladder tumors submitted to surgical removal in 1999, 2000 and 2001. He then embarked upon the use of IP6 as a dietary supplement and has not experienced a return of bladder tumors in 38 months.
And another with images: Cancer and IP6 - Conners Clinic
IP6 enhances the anti-cancer effects of Adriamycin and Tamoxifen, two commonly used cancer drugs. [Tantivejkul K, Breast Cancer Research Treatment 79: 301—12, 2003] However, it goes ignored by cancer doctors even though it’s known to help chemotherapy!
Good article: IP6 is Highly Effective Alternative Treatment for Cancer
IP6 is found effective against prostate cancer
In a recent study at the University of Colorado Cancer Center, more specific mechanisms through which IP6 is effective were identified. Scientists had previously found that IP6 increased the activity of genes that control proteins in human prostate cancer cells lacking functional p53, the gene that provides much cancer protection. They sought to determine whether this increased activity plays a role in IP6's anti-tumor effect. They found that the two genes activated by IP6, p21 and p27, play a critical role in mediating the anticancer effectiveness of IP6. Following activation of the genes by IP6, they were able to halt tumor growth and promote the appropriate death of cells in a process known as apoptosis. This study was published in the February edition of Cancer Research.
This study follows on the heels of other research from the University of Colorado that evaluated the efficacy of IP6 against prostate tumor growth and progression. Prostate cancer was induced in mice given either water containing IP6 or plain water. The researchers found that IP6 inhibited the progression of the cancer cells and strongly reduced the incidence of adenocarcinoma. This is of high significance because 95% of prostate cancers are adenocarcinomas, meaning the cancer has developed in the lining or inner surface of the gland.
The incidences of well-differentiated and poorly differentiated adenocarcinomas in the group fed IP6 were reduced by 44% and 62% respectively. Analysis of the prostate tissue showed a 3.5 fold increase in malignant cell death. This highly significant finding established for the first time that oral IP6 suppresses prostate tumor growth and progression at the stage of abnormal or uncontrolled growth.
Populations with diets high in IP6 have low incidences of cancers
Dr. Shamsuddin's studies have shown that tumor regression takes place in people using 8 grams of IP6 daily for three to four weeks. This is the amount that would be found in 12 ounces of whole kernel corn. One of his studies found that after two weeks the tumors of mice treated three times a week with IP6 were 96 percent smaller than the tumors of mice that did not receive IP6. Populations with diets high in IP6 have lower incidences of cancers of the breast, colon and prostate. Dr. Shamsuddin's laboratory experiments with IP6 have been reproduced and extended by scientists around the world, reconfirming his amazing findings. This study is from Acta Poloniae Pharmaceutica, November through December, 2008.
IP6 is more than a cancer treatment
In addition to the anti-cancer benefits of IP6, research is revealing its benefits in treating diabetes, depression, osteoporosis, heart disease, and kidney stones. It has recently been shown to help Parkinson's patients because of its ability to chelate excess iron and thereby reduce oxidative stress that results in neuronal degradation.
Alot of research on IP6 comes from Dr. Shamsuddin. Here's an interview article about him:
https://www.healthquestpodcast.com/...-6-my-interview-with-dr-abulkalam-shamsuddin/
Quote from the article:
Inositol hexaphosphate (IP(6)) is a naturally occurring carbohydrate, abundantly present in many plant sources and in certain high-fiber diets, such as cereals and legumes. In addition to being found in plants, IP(6) is contained in almost all mammalian cells, although in much smaller amounts, where it is important in regulating vital cellular functions such as signal transduction, cell proliferation, and differentiation. For a long time IP(6) has been recognized as a natural antioxidant. Recently IP(6) has received much attention for its role in cancer prevention and control of experimental tumor growth, progression, and metastasis. In addition, IP(6) possesses other significant benefits for human health, such as the ability to enhance immune system, prevent pathological calcification and kidney stone formation, lower elevated serum cholesterol, and reduce pathological platelet activity. IP(6) is rapidly taken up into cells which further affect signal pathways resulting in cell cycle arrest. A striking anticancer action of IP(6) was demonstrated in different experimental models. In addition to reducing cell proliferation, IP(6) also induces differentiation of malignant cells. Enhanced immunity and antioxidant properties also contribute to tumor cell destruction. Preliminary studies in humans show that IP(6) and inositol, the precursor molecule of IP(6), appear to enhance the anticancer effect of conventional chemotherapy, control cancer metastases, and improve quality of life. Because it is abundantly present in regular diet, efficiently absorbed from the gastrointestinal tract, and safe, IP(6) + inositol holds great promise in our strategies for cancer prevention and therapy. Excerpt (Abstract: Protection against cancer by dietary IP6 and inositol. Nutr Cancer. 2006;55(2):109-25.)
About Dr. Abulkalam Shamsuddin M.B.B.S., Ph.D.
Dr Shamsuddin graduated Dhaka Medical College, University of Dhaka in 1972. Following Internship in Massachusetts and Residency training in pathology in Maryland, he was certified by the American Board of Pathology in 1977. In 1980, he received Ph.D. degree from the University of Maryland for his work on colon carcinogenesis. He joined the faculty of the University of Maryland as an Instructor in 1977, and rose through the ranks of Assistant Professor and Associate Professor to become a full Professor in 1988. For his excellence in teaching he received the Best Teacher award from the medical students many times, including the coveted “Golden Apple Award” by the American Medical Students’ Association in 1999.
Research Interests:
Dr. Shamsuddin has been studying the process of cancer formation and the prevention of cancer since 1975. His research in the steps of colon cancer formation resulted in the development of a very simple and accurate screening test for colon cancer. This rather inexpensive test detects cancers at a very early stage, and even before they have formed; in other words, it can also detect precancerous polyps and other precancerous lesions and conditions. The test has been in use in China since the early 1990’s often referred to as “Shams’ Test.” He further discovered that the colon cancer marker Gal-GalNAc is a common cancer marker expressed through a similar “field-effect” phenomenon; thus forming the basis for a general cancer screening test such as for cancer of the lungs and breast, and possibly prostate and uterine cervix.
In mid 1985, Dr. Shamsuddin started his pioneering experiments on the anti-cancer property of inositol and inositol hexaphosphate (IP-6) – natural constituents of cereals and legumes such as rice, corn, beans etc. For the next two decades, he has reconfirmed and expanded his groundbreaking studies to show the efficacy of IP-6 and inositol against different cancers in various experimental models. Dr. Shamsuddin’s book on this “IP6: Nature’s Revolutionary Cancer Fighter” by Kensington Publishing Corporation, New York, 1998, is written for the general public. Aside from these Dr. Shamsuddin has contributed numerous book-chapters and published over 200 scientific papers.
IP6 is apparently effective when combined in a good ratio with inositol, creating IP3. IP3 is very potent anti-cancer, sources state.
Here is an informative video on IP6:
And another one:
IP6 with inositol is found naturally in every body cell in the human body according to Dr. Shamsuddin. It is disputed wether or not IP6 plays a negative role in radiation therapy for cancer, as IP6 appears to stimulate the NHEJ pathway, according to the following article publishedin 2004, with its discovery of IP6 stimulating NHEJ in 2000:
Enemy Within
Dr. Shamsuddin says however, in the following article which was published in 2007 that it is protective and synergetic in radiation therapy for cancer, and that he was inspired to research this as reports from a trial in Croatia which begun in 2001 showed these protective effects during radiation therapy:
https://www.sciencedaily.com/releases/2007/11/071105083735.htm
So all in all, its role concerning radiation and radiation therapy for cancer should be further investigated, as it is disputed.
Also an informative website, by Dr. Shamsuddin himself, I think: http://www.ip-6.net/The_Science.html
So all in all, IP6 seems to be a very interesting molecule.
What do you know about IP6?
Last edited:

