DaveFoster
Member
Dr. Peat has favorably commented on methylphenidate (Ritalin or Concerta) in the context of ADHD, and he has recommended coffee (due to the caffeine) as an alternative for ADHD medication. The following studies explore some of the hormonal effects of methylphenidate.
The first study on mice at 8 weeks old (the equivalent of around 20 years in human age) used a human dosage equivalent of about 10 mg daily for a 60 kg person. It showed that methylphenidate abolishes testosterone levels, impairs fertility and reduces the number of Leydig cells. The higher dose of 50 mg (in the human-equivalent dosage) actually lowered testosterone less than the smaller dosage, which implies that methylphenidate supports testosterone production, possibly through its dopaminergic (dopamine-like) effects. The researchers posited many possible avenues for methylphenidate's testosterone-lowering effects, but one of the most obvious suggested the drug's anoretic (appetite-reducing) effects and subsequent lowered caloric intakes.

"In the present study, the weights of mice in the treatment groups showed significant reduction in comparison with those in control groups. In another study, the mice receiving hydrochloride -MPH similarly showed a significant reduction in body weight (17). In fact, body weight reduction could be due to decrease of appetite. Some other chemicals, such as amphetamine could also cause body weight reduction. Weight loss after MPH treatment could be due to several factors, such as anorexia, stomach cramps, or insomnia, that reduce food intake. Weight loss caused by reduced food intake can severely affect growth and pubertal development by perturbing the maturation of not only the hypothalamo –pituitary– gonadal axis but also the axes of the hypothalamus and pituitary with the adrenals, thyroid, and growth hormone. Other nonspecific effects of weight loss that might indirectly affect growth and pubertal development include the following: gastrointestinal complications of reduced taste, delayed gastric emptying, or malabsorption (6). Therefore, the same reasons might be responsible for body weight reduction following MPH administration. It was observed that chronic oral use of MPH did not bring about any changes in weight or size of testes. However, a study on rats has shown that gavages of hydrochloride cocaine (15 mg/kg), which is structurally similar to MPH, prescribed for 100 days, resulted in testicular weight reduction (12). This study showed the significant reduction of the number of leydig cells in the treatment groups compared with the control group. Some other reports, confirming this finding, have indicated that intraperitoneal injection of hydrochloride cocaine at a dose of 30 mg/kg could cause necrosis and also decrease in the number of interstitial cells of the testes (18).
Serum Testosterone secretion showed a significant reduction in the treatment groups of this study. A similar study on the short term effects of MPH on the production of sex-hormone in male mice showed that exposure of mice to MPH causes considerable reduction in testosterone production..." (Fazelipour, et al., 2012).
The second study studied the effect of methylphenidate on testosterone when administered acutely for a duration of only 4 weeks to older children with ADHD and found no change in testosterone levels.
The third study, done on adolescent primates, rhesus monkeys, showed that methylphenidate reduced testosterone, shrunk the testicles and delayed puberty. Interestingly, the effects reversed over time.
Researchers experimented on 80 rats total. They separated 40 into a regular cage and another 40 in an enriched (ie, stimulating) environment. They gave 20 from one group methylphenidate, and then gave the drug to 20 of the other group. They then took 10 rats from each of the four groups of 20 (so half of all rats) and exposed them to stress, and they did not expose the other half. The figure below taken from the study simplifies how their methods.
The saline groups were injected with saline just to mimic the psychological effects of being picked up and injected with a needle; the saline had minimal effects, so those were the "control groups," or the rats not given methylphenidate. "PND" just means days along in the study.
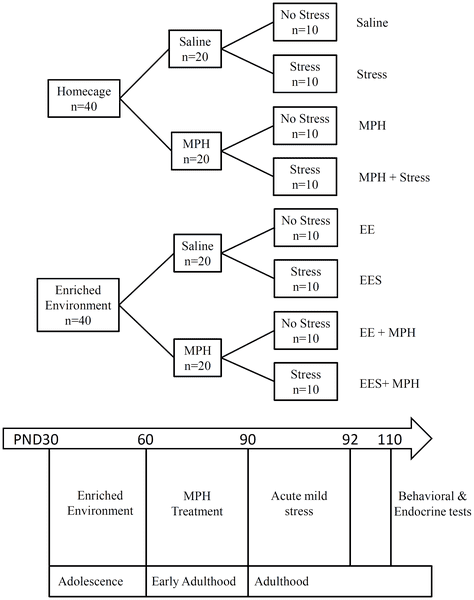
Figure 1. Schematic diagram of experimental design and procedures.
Methylphenidate increased both testosterone and corticosterone in all cases, but the ratio of testosterone to corticosterone determines the level of physiological stress.
They found that, for a dosage of about 22 mg daily for a 60 kg person, methylphenidate muffled the hormonal anti-stress effects of a positive stimulating environment without stress (a bad effect), did not reduce the effects of stress after exposure to a stimulating environment (a neutral effect), and abolished the negative effects of stress (a great effect).
Methylphenidate amplified the reductions of rat movement in a stimulating environment; the lessened movement indicates lowered stress, so methylphenidate increases the behavioral manifestations of an anti-stress environment.
Methylphenidate also lowered body weight the most for any group except for that injected with the saline solution, and the drug also enhanced sugar cravings.
See Figure 2 taken from the study to represent the aforementioned behavioral effects of methylphenidate, Figure 3 to illustrate the difference in sugar cravings and see Figure 5 for the hormonal effects of the drug.
"Attention-deficit/hyperactivity disorder (ADD/ADHD) has been emerging as a world-wide psychiatric disorder. There appears to be an increasing rate of stimulant drug abuse, specifically methylphenidate (MPH) which is the most common treatment for ADHD, among individuals who do not meet the criteria for ADHD and particularly for cognitive enhancement among university students. However, the long term effects of exposure to MPH are unknown. Thus, in light of a developmental approach in humans, we aimed to test the effects of adolescence exposure to enriched environment (EE) followed by MPH administration during early adulthood, on reactions to stress in adulthood. Specifically, at approximate adolescence [post natal days (PND) 30–60] rats were reared in EE and were treated with MPH during early adulthood (PND 60–90). Adult (PND 90–92) rats were exposed to mild stress and starting at PND 110, the behavioral and endocrine effects of the combined drug and environmental conditions were assessed. Following adolescence EE, long term exposure to MPH led to decreased locomotor activity and increased sucrose preference. EE had a beneficial effect on PPI (attentive abilities), which was impaired by long term exposure to MPH. Finally, the interaction between EE and, exposure to MPH led to long-term elevated corticosterone and testosterone levels. In view of the marked increase in MPH consumption over the past decade, vigilance is crucial in order to prevent potential drug abuse and its long term detrimental consequences.
...A significant difference in locomotor activity measured by distance (Figure 2A) and velocity (Figure 2B) was detected between the groups [F(3, 71) = 25.4, P<0.0001)]. We found that adolescence EE led to a long term decrease in distance and velocity (P<0.0001). Surprisingly, MPH administration following adolescence EE intensifies these effects over time (P<0.022; P<0.008, respectively). Interestingly, the exposure to a brief stress (EE+Stress) in adulthood moderates these alterations. In order to exclude the effect of body weight on locomotor behavior, indeed an insignificant difference in body weight gain (Figure 2C) between all groups was found [F(7, 71)<1].

Figure 2. The effect of MPH on locomotor activity.
A significant difference in distance (A) and velocity (B) were detected between the groups in the open field test. Rats exposed to MPH following EE showed the lowest distance and velocity. These effects were not related to differences in body weight (C). MPH treatment significantly increased freezing duration (D) compared to saline. Following EE, MPH led to the highest freezing duration. * P<0.0001 versus control; ** a: P<0.022, b: P<0.008, c: P<0.0001 versus EE saline; n = 9 to 10 per group.
Figure 2D), a significant difference was detected between the groups [F(3, 71) = 75.83, P<0.0001]. The highest freezing level was detected in the EE group (P<0.0001), while the EE followed by stress (EES) demonstrated a significant increase compared to both control and stress groups (P<0.0001). In addition, MPH was associated with significantly higher freezing in both control (P<0.001), EE (P<0.0001) and EES (P<0.006) groups.
...A significant diversity in sucrose preference was observed between the groups [F(3, 71) = 31.57, P<0.0001]. Complementarily to the highest freezing level observed in the EE group, a significant long term anhedonia was detected (P<0.019). Fascinatingly, MPH administration has led to anhedonia in the controls (P<0.001), while in the EE group MPH significantly shifted to increased hedonia (P<0.0001). Finally, the EES group showed a significant hedonia (P<0.0001) as compared with either the EE or stress groups, that was not affected by MPH administration.
 Figure 3. The effect of MPH on sucrose preference test.
Figure 3. The effect of MPH on sucrose preference test.
Considerable variation in sucrose intake was observed between the groups. While significant long term anhedonia was detected in the EE group, MPH treatment following EE significantly recovers this effect. * P<0.019 versus control; ** P<0.0001 versus EE saline; n = 9 to 10 per group.
MPH detrimental effects on Pre-Pulse Inhibition following adolescence EE or preceding stress in adulthood
Pre-Pulse Inhibition test (PPI) is a neurological phenomenon (measured also in human subjects) in which a weaker acoustic pre-pulse, inhibits the reaction to a subsequent strong startling pulse. The reduction of the amplitude of response reflects the ability of the nervous system to temporarily adapt to a strong sensory stimulus when a preceding weaker signal is given. We found (Figure 4) a significant distinction in PPI along different pre- intensities (59 db to 85 db) across all groups [F(6, 30) = 459.42, P<0.0001) and between the groups [F(3, 35) = 161.12, P<0.0001]. The exposure to adolescence EE has led to a long term beneficial effect on PPI (P<0.0001), while stress following EE has lessened this increase (P<0.019). When administrated following EE (EE+MPH) or prior to stress (MPH+stress), MPH had a long term deteriorating effect on PPI (P<0.022; P<0.004, respectively). However, by itself (MPH group) or in the EES group, MPH seemed to have only minor effect.

Figure 4. The effect of MPH on PPI.
Significant differences in PPI scores were observed. EE led to the highest PPI compared with all groups (P<0.0001). Following MPH treatment, the EES group showed higher PPI compared with control (P<0.0001) and stress (P<0.001) groups. A panel of representative traces demonstrate the differences in maximal response inhibition (at pre-intensity of 69 dB) of all four groups, with and without MPH. n = 9 to 10 per group.
...In trying to depict the endocrine mechanism mediating the effects of MPH in the context of the developmental approach, previous studies found that EE lowered baseline corticosterone (CORT) level [38] or did not influence its level at all [39], while MPH (similar to hyper-arousal following stress) was found to increase CORT level [30], [33], [40], [41]. To clarify the aforementioned behavioral effects, we measured serum CORT level (Figure 5A) and found a significant difference between the groups [F(3, 56) = 51.019, P<0.0001]. Without MPH, a long term significant CORT reduction was found in the EE group (P<0.026) while in the stress and the EES groups CORT was elevated (P<0.004). Interestingly, MPH administration increased CORT level (more than 152%) in the EE (P<0.0001) and EES (P<0.002) groups.
 Figure 5. The effect of MPH on CORT and TST levels.
Figure 5. The effect of MPH on CORT and TST levels.
A significant difference in CORT (A) and TST (B) levels was observed. MPH treatment significantly increased CORT level in the EE and EES groups (* P<0.0001; ** P<0.002 compared with their respective controls). TST level was similar across all groups, while MPH treatment increased TST level in both EE and EES groups (* P<0.001; ** P<0.012 compared with their counterpart controls). n = 9 to 10 per group.
[42], [43]. Specifically, it has been suggested that children with ADHD generate more aggressive responses to provocation and that this may be exacerbated by administration of MPH [42]. This led us to examine the endocrine correlate of aggression, by measuring Testosterone (TST) serum level [44]–[47]. A significant variation in TST level (Figure 5B) was observed between the groups [F(3, 56) = 14.12, P<0.0001]. Surprisingly, we found a robust long-term increase (more than 154%) in the EE and EES groups treated with MPH (P<0.001 and P<0.012 respectively)" (Avital, et al., 2011).
PLOS link: Environmental Enrichment Preceding Early Adulthood Methylphenidate Treatment Leads to Long Term Increase of Corticosterone and Testosterone in the Rat
References
Avital, Avi, et al. “Environmental Enrichment Preceding Early Adulthood Methylphenidate Treatment Leads to Long Term Increase of Corticosterone and Testosterone in the Rat.” PLoS ONE, vol. 6, no. 7, July 2011. PubMed Central, doi:10.1371/journal.pone.0022059.
Fazelipour, Simin, et al. “The Effect of Chronic Administration of Methylphenidate on Morphometric Parameters of Testes and Fertility in Male Mice.” Journal of Reproduction & Infertility, vol. 13, no. 4, 2012, pp. 232–36.
Mattison, Donald R., et al. “Pubertal Delay in Male Nonhuman Primates (Macaca Mulatta) Treated with Methylphenidate.” Proceedings of the National Academy of Sciences of the United States of America, vol. 108, no. 39, Sept. 2011, pp. 16301–06. PubMed Central, doi:10.1073/pnas.1102187108.
Wang, Liang-Jen, et al. “Does Methylphenidate Reduce Testosterone Levels in Humans? A Prospective Study in Children with Attention-Deficit/Hyperactivity Disorder.” International Journal of Neuropsychopharmacology, vol. 20, no. 3, Dec. 2016, pp. 219–27. PubMed Central, doi:10.1093/ijnp/pyw101.
The first study on mice at 8 weeks old (the equivalent of around 20 years in human age) used a human dosage equivalent of about 10 mg daily for a 60 kg person. It showed that methylphenidate abolishes testosterone levels, impairs fertility and reduces the number of Leydig cells. The higher dose of 50 mg (in the human-equivalent dosage) actually lowered testosterone less than the smaller dosage, which implies that methylphenidate supports testosterone production, possibly through its dopaminergic (dopamine-like) effects. The researchers posited many possible avenues for methylphenidate's testosterone-lowering effects, but one of the most obvious suggested the drug's anoretic (appetite-reducing) effects and subsequent lowered caloric intakes.
"In the present study, the weights of mice in the treatment groups showed significant reduction in comparison with those in control groups. In another study, the mice receiving hydrochloride -MPH similarly showed a significant reduction in body weight (17). In fact, body weight reduction could be due to decrease of appetite. Some other chemicals, such as amphetamine could also cause body weight reduction. Weight loss after MPH treatment could be due to several factors, such as anorexia, stomach cramps, or insomnia, that reduce food intake. Weight loss caused by reduced food intake can severely affect growth and pubertal development by perturbing the maturation of not only the hypothalamo –pituitary– gonadal axis but also the axes of the hypothalamus and pituitary with the adrenals, thyroid, and growth hormone. Other nonspecific effects of weight loss that might indirectly affect growth and pubertal development include the following: gastrointestinal complications of reduced taste, delayed gastric emptying, or malabsorption (6). Therefore, the same reasons might be responsible for body weight reduction following MPH administration. It was observed that chronic oral use of MPH did not bring about any changes in weight or size of testes. However, a study on rats has shown that gavages of hydrochloride cocaine (15 mg/kg), which is structurally similar to MPH, prescribed for 100 days, resulted in testicular weight reduction (12). This study showed the significant reduction of the number of leydig cells in the treatment groups compared with the control group. Some other reports, confirming this finding, have indicated that intraperitoneal injection of hydrochloride cocaine at a dose of 30 mg/kg could cause necrosis and also decrease in the number of interstitial cells of the testes (18).
Serum Testosterone secretion showed a significant reduction in the treatment groups of this study. A similar study on the short term effects of MPH on the production of sex-hormone in male mice showed that exposure of mice to MPH causes considerable reduction in testosterone production..." (Fazelipour, et al., 2012).
Pubmed link: The Effect of Chronic Administration of Methylphenidate on Morphometric Parameters of Testes and Fertility in Male Mice
The second study studied the effect of methylphenidate on testosterone when administered acutely for a duration of only 4 weeks to older children with ADHD and found no change in testosterone levels.
"Animal studies and case reports have suggested that methylphenidate exerts adverse effects on gonadal hormones. This study aimed to determine whether methylphenidate alters testosterone levels in children with attention-deficit/hyperactivity disorder through comparison of those with or without methylphenidate treatment.
This 4-week, nonrandomized, prospective study conducted in Taiwan included 203 attention-deficit/hyperactivity disorder patients with a mean age of 8.7 years (boys: 75.8%). After the initial recruitment, 137 received daily methylphenidate treatment (medicated group) and 66 were assessed through naturalistic observation (nonmedicated group). The saliva samples of attention-deficit/hyperactivity disorder patients were used to quantify testosterone levels at baseline and the endpoint by using the chemiluminescence immunoassay. At the 4th week, 86 patients in the medicated group and 46 patients in the nonmedicated group were eligible for statistical analyses.
During the study period, salivary testosterone levels did not significantly change in the medicated group (P=.389) or in the nonmedicated group (P=.488). After correction for the potential confounding effects of age and sex, salivary testosterone levels still remained unchanged in the medicated and nonmedicated groups during the 4-week follow-up. In the medicated group, changes in salivary testosterone levels over 4 weeks were not significantly correlated with the methylphenidate daily dose (mean daily dose: 18.1 mg).
Findings suggest that short-term treatment with methylphenidate at usual doses does not significantly alter salivary testosterone levels in attention-deficit/hyperactivity disorder patients. Future studies should clarify whether long-term methylphenidate treatment disrupts testosterone production as well as the function of the reproductive system" (Wang, et al., 2016).
PubMed link: Does Methylphenidate Reduce Testosterone Levels in Humans? A Prospective Study in Children with Attention-Deficit/Hyperactivity Disorder
This 4-week, nonrandomized, prospective study conducted in Taiwan included 203 attention-deficit/hyperactivity disorder patients with a mean age of 8.7 years (boys: 75.8%). After the initial recruitment, 137 received daily methylphenidate treatment (medicated group) and 66 were assessed through naturalistic observation (nonmedicated group). The saliva samples of attention-deficit/hyperactivity disorder patients were used to quantify testosterone levels at baseline and the endpoint by using the chemiluminescence immunoassay. At the 4th week, 86 patients in the medicated group and 46 patients in the nonmedicated group were eligible for statistical analyses.
During the study period, salivary testosterone levels did not significantly change in the medicated group (P=.389) or in the nonmedicated group (P=.488). After correction for the potential confounding effects of age and sex, salivary testosterone levels still remained unchanged in the medicated and nonmedicated groups during the 4-week follow-up. In the medicated group, changes in salivary testosterone levels over 4 weeks were not significantly correlated with the methylphenidate daily dose (mean daily dose: 18.1 mg).
Findings suggest that short-term treatment with methylphenidate at usual doses does not significantly alter salivary testosterone levels in attention-deficit/hyperactivity disorder patients. Future studies should clarify whether long-term methylphenidate treatment disrupts testosterone production as well as the function of the reproductive system" (Wang, et al., 2016).
PubMed link: Does Methylphenidate Reduce Testosterone Levels in Humans? A Prospective Study in Children with Attention-Deficit/Hyperactivity Disorder
The third study, done on adolescent primates, rhesus monkeys, showed that methylphenidate reduced testosterone, shrunk the testicles and delayed puberty. Interestingly, the effects reversed over time.
"Juvenile male rhesus monkeys treated with methylphenidate hydrochloride (MPH) to evaluate genetic and behavioral toxicity were observed after 14 mo of treatment to have delayed pubertal progression with impaired testicular descent and reduced testicular volume. Further evaluation of animals dosed orally twice a day with (i) 0.5 mL/kg of vehicle (n = 10), (ii) 0.15 mg/kg of MPH increased to 2.5 mg/kg (low dose, n = 10), or (iii) 1.5 mg/kg of MPH increased to 12.5 mg/kg (high dose, n = 10) for a total of 40 mo revealed that testicular volume was significantly reduced (P < 0.05) at months 15 to 19 and month 27. Testicular descent was significantly delayed (P < 0.05) in the high-dose group. Significantly lower serum testosterone levels were detected in both the low- (P = 0.0017) and high-dose (P = 0.0011) animals through month 33 of treatment. Although serum inhibin B levels were increased overall in low-dose animals (P = 0.0328), differences between groups disappeared by the end of the study. Our findings indicate that MPH administration, beginning before puberty, and which produced clinically relevant blood levels of the drug, impaired pubertal testicular development until ∼5 y of age. It was not possible to resolve whether MPH delayed the initiation of the onset of puberty or reduced the early tempo of the developmental process. Regardless, deficits in testicular volume and hormone secretion disappeared over the 40-mo observation period, suggesting that the impact of MPH on puberty is not permanent" (Mattison, et al., 2011).
PubMed link: Pubertal delay in male nonhuman primates (Macaca mulatta) treated with methylphenidate. - PubMed - NCBI
The fourth and final study, by far the most interesting, measured the impacts of methylphenidate on stress. Note that corticosterone in rats can be used to represent the stress hormone cortisol in humans. PubMed link: Pubertal delay in male nonhuman primates (Macaca mulatta) treated with methylphenidate. - PubMed - NCBI
Researchers experimented on 80 rats total. They separated 40 into a regular cage and another 40 in an enriched (ie, stimulating) environment. They gave 20 from one group methylphenidate, and then gave the drug to 20 of the other group. They then took 10 rats from each of the four groups of 20 (so half of all rats) and exposed them to stress, and they did not expose the other half. The figure below taken from the study simplifies how their methods.
The saline groups were injected with saline just to mimic the psychological effects of being picked up and injected with a needle; the saline had minimal effects, so those were the "control groups," or the rats not given methylphenidate. "PND" just means days along in the study.
Figure 1. Schematic diagram of experimental design and procedures.
They found that, for a dosage of about 22 mg daily for a 60 kg person, methylphenidate muffled the hormonal anti-stress effects of a positive stimulating environment without stress (a bad effect), did not reduce the effects of stress after exposure to a stimulating environment (a neutral effect), and abolished the negative effects of stress (a great effect).
Methylphenidate amplified the reductions of rat movement in a stimulating environment; the lessened movement indicates lowered stress, so methylphenidate increases the behavioral manifestations of an anti-stress environment.
Methylphenidate also lowered body weight the most for any group except for that injected with the saline solution, and the drug also enhanced sugar cravings.
See Figure 2 taken from the study to represent the aforementioned behavioral effects of methylphenidate, Figure 3 to illustrate the difference in sugar cravings and see Figure 5 for the hormonal effects of the drug.
"Attention-deficit/hyperactivity disorder (ADD/ADHD) has been emerging as a world-wide psychiatric disorder. There appears to be an increasing rate of stimulant drug abuse, specifically methylphenidate (MPH) which is the most common treatment for ADHD, among individuals who do not meet the criteria for ADHD and particularly for cognitive enhancement among university students. However, the long term effects of exposure to MPH are unknown. Thus, in light of a developmental approach in humans, we aimed to test the effects of adolescence exposure to enriched environment (EE) followed by MPH administration during early adulthood, on reactions to stress in adulthood. Specifically, at approximate adolescence [post natal days (PND) 30–60] rats were reared in EE and were treated with MPH during early adulthood (PND 60–90). Adult (PND 90–92) rats were exposed to mild stress and starting at PND 110, the behavioral and endocrine effects of the combined drug and environmental conditions were assessed. Following adolescence EE, long term exposure to MPH led to decreased locomotor activity and increased sucrose preference. EE had a beneficial effect on PPI (attentive abilities), which was impaired by long term exposure to MPH. Finally, the interaction between EE and, exposure to MPH led to long-term elevated corticosterone and testosterone levels. In view of the marked increase in MPH consumption over the past decade, vigilance is crucial in order to prevent potential drug abuse and its long term detrimental consequences.
...A significant difference in locomotor activity measured by distance (Figure 2A) and velocity (Figure 2B) was detected between the groups [F(3, 71) = 25.4, P<0.0001)]. We found that adolescence EE led to a long term decrease in distance and velocity (P<0.0001). Surprisingly, MPH administration following adolescence EE intensifies these effects over time (P<0.022; P<0.008, respectively). Interestingly, the exposure to a brief stress (EE+Stress) in adulthood moderates these alterations. In order to exclude the effect of body weight on locomotor behavior, indeed an insignificant difference in body weight gain (Figure 2C) between all groups was found [F(7, 71)<1].
Figure 2. The effect of MPH on locomotor activity.
A significant difference in distance (A) and velocity (B) were detected between the groups in the open field test. Rats exposed to MPH following EE showed the lowest distance and velocity. These effects were not related to differences in body weight (C). MPH treatment significantly increased freezing duration (D) compared to saline. Following EE, MPH led to the highest freezing duration. * P<0.0001 versus control; ** a: P<0.022, b: P<0.008, c: P<0.0001 versus EE saline; n = 9 to 10 per group.
Figure 2D), a significant difference was detected between the groups [F(3, 71) = 75.83, P<0.0001]. The highest freezing level was detected in the EE group (P<0.0001), while the EE followed by stress (EES) demonstrated a significant increase compared to both control and stress groups (P<0.0001). In addition, MPH was associated with significantly higher freezing in both control (P<0.001), EE (P<0.0001) and EES (P<0.006) groups.
...A significant diversity in sucrose preference was observed between the groups [F(3, 71) = 31.57, P<0.0001]. Complementarily to the highest freezing level observed in the EE group, a significant long term anhedonia was detected (P<0.019). Fascinatingly, MPH administration has led to anhedonia in the controls (P<0.001), while in the EE group MPH significantly shifted to increased hedonia (P<0.0001). Finally, the EES group showed a significant hedonia (P<0.0001) as compared with either the EE or stress groups, that was not affected by MPH administration.
Considerable variation in sucrose intake was observed between the groups. While significant long term anhedonia was detected in the EE group, MPH treatment following EE significantly recovers this effect. * P<0.019 versus control; ** P<0.0001 versus EE saline; n = 9 to 10 per group.
MPH detrimental effects on Pre-Pulse Inhibition following adolescence EE or preceding stress in adulthood
Pre-Pulse Inhibition test (PPI) is a neurological phenomenon (measured also in human subjects) in which a weaker acoustic pre-pulse, inhibits the reaction to a subsequent strong startling pulse. The reduction of the amplitude of response reflects the ability of the nervous system to temporarily adapt to a strong sensory stimulus when a preceding weaker signal is given. We found (Figure 4) a significant distinction in PPI along different pre- intensities (59 db to 85 db) across all groups [F(6, 30) = 459.42, P<0.0001) and between the groups [F(3, 35) = 161.12, P<0.0001]. The exposure to adolescence EE has led to a long term beneficial effect on PPI (P<0.0001), while stress following EE has lessened this increase (P<0.019). When administrated following EE (EE+MPH) or prior to stress (MPH+stress), MPH had a long term deteriorating effect on PPI (P<0.022; P<0.004, respectively). However, by itself (MPH group) or in the EES group, MPH seemed to have only minor effect.
Figure 4. The effect of MPH on PPI.
Significant differences in PPI scores were observed. EE led to the highest PPI compared with all groups (P<0.0001). Following MPH treatment, the EES group showed higher PPI compared with control (P<0.0001) and stress (P<0.001) groups. A panel of representative traces demonstrate the differences in maximal response inhibition (at pre-intensity of 69 dB) of all four groups, with and without MPH. n = 9 to 10 per group.
...In trying to depict the endocrine mechanism mediating the effects of MPH in the context of the developmental approach, previous studies found that EE lowered baseline corticosterone (CORT) level [38] or did not influence its level at all [39], while MPH (similar to hyper-arousal following stress) was found to increase CORT level [30], [33], [40], [41]. To clarify the aforementioned behavioral effects, we measured serum CORT level (Figure 5A) and found a significant difference between the groups [F(3, 56) = 51.019, P<0.0001]. Without MPH, a long term significant CORT reduction was found in the EE group (P<0.026) while in the stress and the EES groups CORT was elevated (P<0.004). Interestingly, MPH administration increased CORT level (more than 152%) in the EE (P<0.0001) and EES (P<0.002) groups.
A significant difference in CORT (A) and TST (B) levels was observed. MPH treatment significantly increased CORT level in the EE and EES groups (* P<0.0001; ** P<0.002 compared with their respective controls). TST level was similar across all groups, while MPH treatment increased TST level in both EE and EES groups (* P<0.001; ** P<0.012 compared with their counterpart controls). n = 9 to 10 per group.
[42], [43]. Specifically, it has been suggested that children with ADHD generate more aggressive responses to provocation and that this may be exacerbated by administration of MPH [42]. This led us to examine the endocrine correlate of aggression, by measuring Testosterone (TST) serum level [44]–[47]. A significant variation in TST level (Figure 5B) was observed between the groups [F(3, 56) = 14.12, P<0.0001]. Surprisingly, we found a robust long-term increase (more than 154%) in the EE and EES groups treated with MPH (P<0.001 and P<0.012 respectively)" (Avital, et al., 2011).
PLOS link: Environmental Enrichment Preceding Early Adulthood Methylphenidate Treatment Leads to Long Term Increase of Corticosterone and Testosterone in the Rat
References
Avital, Avi, et al. “Environmental Enrichment Preceding Early Adulthood Methylphenidate Treatment Leads to Long Term Increase of Corticosterone and Testosterone in the Rat.” PLoS ONE, vol. 6, no. 7, July 2011. PubMed Central, doi:10.1371/journal.pone.0022059.
Fazelipour, Simin, et al. “The Effect of Chronic Administration of Methylphenidate on Morphometric Parameters of Testes and Fertility in Male Mice.” Journal of Reproduction & Infertility, vol. 13, no. 4, 2012, pp. 232–36.
Mattison, Donald R., et al. “Pubertal Delay in Male Nonhuman Primates (Macaca Mulatta) Treated with Methylphenidate.” Proceedings of the National Academy of Sciences of the United States of America, vol. 108, no. 39, Sept. 2011, pp. 16301–06. PubMed Central, doi:10.1073/pnas.1102187108.
Wang, Liang-Jen, et al. “Does Methylphenidate Reduce Testosterone Levels in Humans? A Prospective Study in Children with Attention-Deficit/Hyperactivity Disorder.” International Journal of Neuropsychopharmacology, vol. 20, no. 3, Dec. 2016, pp. 219–27. PubMed Central, doi:10.1093/ijnp/pyw101.
